Docket #: S14-465
Low Cost, Bifunctional Electrocatalyst for Water Splitting
Stanford researchers have developed and tested a low cost, bifunctional water splitting catalyst that outperforms conventional catalysts. The inexpensive Ni3FeOx nanoparticle facilitates both the oxygen and hydrogen evolution reactions, simplifying electrolysis deployment and scale up. The approach improves transition metal oxides/chalcogenides performance in reactions crucial to renewable energy production such as hydrogen production, CO2 reduction, and methane oxidation. This inexpensive catalyst could make water splitting, renewable energy production, rechargeable metal-air batteries and fuel cells commercially viable.
Stage of Research
Researchers tested various nanoparticle morphologies of the bifunctional electrocatalyst (applied to carbon fiber paper) against conventional catalysts, Pt and Ir. With the input of a 1.5V battery the bi-functional catalyst split water continuously for a week at 82% efficiency. The conventional catalyst performance degraded to 65% efficiency after only 24 hours.
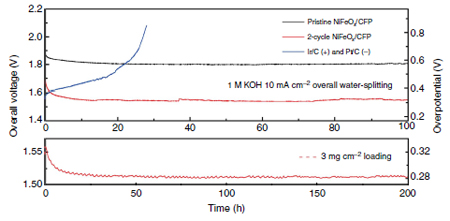
Figure 1 Catalyst performance comparison of conventional Ir and Pt, and Ni3FeOx catalysts.
The 2-cycle catalyst has more grain boundaries, more active reaction sites.
Applications
- Catalysts for end user applications in:
- Water splitting / hydrogen production
- Oxygen and hydrogen reduction reactions - fuel cells, supercapacitors
- CO2 reduction – recycling CO2 into reusable fuels
- Methane oxidation – reducing greenhouse gas emission
Advantages
- Low cost – inexpensive single catalyst, readily available, easy to deploy and scale up
- Efficient – 82% efficiency (at a constant 1.5V) for over a week
Publications
- H. Wang, H.-W. Lee, Y. Deng, Z. Lu, P.-C. Hsu, Y. Liu, D. Lin, and Y. Cui, "Bifunctional non-noble metal oxide nanoparticle electrocatalysts through lithium-induced conversion for overall water splitting." Nature Comm. 6, 7261 (2015) doi:10.1038/ncomms8261
- Stanford University. "Single-catalyst water splitter produces clean-burning hydrogen 24/7." ScienceDaily. ScienceDaily, 23 June 2015.
Related Links
Similar Technologies
-
Electro-thermochemical Li Cycling for NH3 Synthesis from N2 and H2O S16-240Electro-thermochemical Li Cycling for NH3 Synthesis from N2 and H2O
-
High efficiency electrocatalysis with lung-inspired architecture S17-342High efficiency electrocatalysis with lung-inspired architecture
-
Simple synthesis of uniform carbon flower superstructures for electrocatalysts and other energy and environment applications S18-114Simple synthesis of uniform carbon flower superstructures for electrocatalysts and other energy and environment applications