Docket #: S22-232
Methodology for tailoring target-specific ultrasound contrast agent production with pre-formed ligand-phospholipid bioconjugates
Stanford researchers have developed a new controllable methodology for molecularly targeted ultrasound contrast agent production with pre-formed ligand-phospholipid bioconjugates. This approach solves the issue of consistency in producing ready-to-use and clinically translatable ultrasound molecular imaging agents targeted against biomarkers representing pathological angiogenesis or abnormal cells present in cancer or inflammatory diseases. Small protein ligands engineered to bind disease biomarkers are used to form ligand-phospholipid conjugates and incorporated into gas-filled contrast agents using conventional production methods or microfluidics-based platforms. This tailored target-specific ultrasound contrast agent production method overcomes the current limitations in producing uniformly targeted microbubbles in a scalable, economical, and reproducible manner.
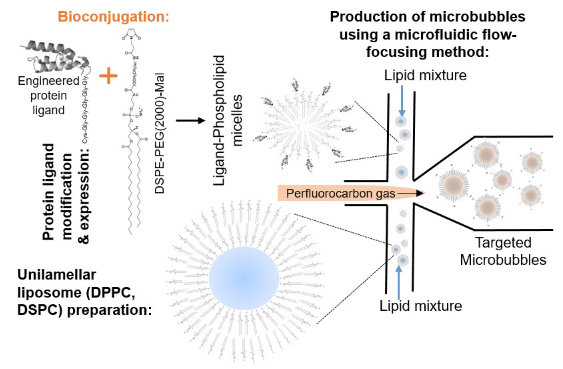
Description: Small protein ligands (example: affibody) engineered to bind to disease specific biomarkers are modified to express a terminal amino acid (example: cysteine) that enable clinically applicable bioconjugation (example: thiol-maleimide chemistry) of ligands to phospholipid micelles. Bioconjugates are mixed with phospholipid liposomal vesicles (example: DPPC, DSPC) and ultrasound responsive gas (example: perfluorobutane) to produce targeted microbubbles by traditional mechanical agitation methods (example: VialMix) or through microfluidic systems with specific chip geometries (example: flow-focusing chip format shown in the image).
Stage of Development
Pre-clinical testing for target binding and contrast signal properties
The microbubbles are being tested in animal models of breast cancer for tumor detection by preclinical and clinical ultrasound imaging systems.
Applications
- Diagnostic: Molecular imaging for early detection or staging of disease (cancer of breast, kidney, etc) with all imaging modalities using designed single or multi-target contrast agents.
- Therapeutic: Targeted delivery of drugs (chemotherapy, immunotherapy) loaded into these targeted imaging agents. Molecular imaging-guided determination of tumor tissue areas for therapy optimization with ionizing radiation or high-intensity focused ultrasound. Therapy response monitoring with molecular imaging.
Advantages
- Targeted - allows for targeted microbubbles against novel disease biomarkers
- Highly controllable and uniform production
- Economical and scalable for clinical applications - uses small engineered proteins, as opposed to bigger molecules such as antibodies, with phospholipids
- Works with all imaging modalities
Related Links
Similar Technologies
-
Ultrasonic neuromodulation with Pattern Interference Radiation Force (PIRF) S16-382Ultrasonic neuromodulation with Pattern Interference Radiation Force (PIRF)
-
Ultrasound for Detecting and Suppressing Epileptic Seizure S18-336Ultrasound for Detecting and Suppressing Epileptic Seizure
-
Wedge Transducer for Transcranial Ultrasound S16-003Wedge Transducer for Transcranial Ultrasound