Docket #: S18-217
Non-invasive blood test for diagnosing Crohn's disease and ulcerative colitis
Researchers at Stanford have developed a non-invasive method, based on the identification of novel immune signatures in the blood, for diagnosing Crohn's disease (CD) or ulcerative colitis (UC) in patients with inflammatory bowel disease (IBD). This diagnosis has conventionally relied on invasive, expensive and risky endoscopic procedures. Misdiagnosis and delayed diagnosis due to time required for endoscopy and histopathology analysis of biopsies remain serious problems that delay optimal treatment representing a critical unmet need. Earlier treatment leads to better outcomes, and treatment for patients with active disease is frequently delayed by the time required for differentiation of CD versus UC by these invasive and costly procedures. In contrast, the new method may be implemented easily on existing equipment using a single fresh or frozen blood specimen, potentially enabling earlier and more definitive diagnosis, as well as with minor adaptations better monitoring and precision treatment of IBD patients.
Figure:
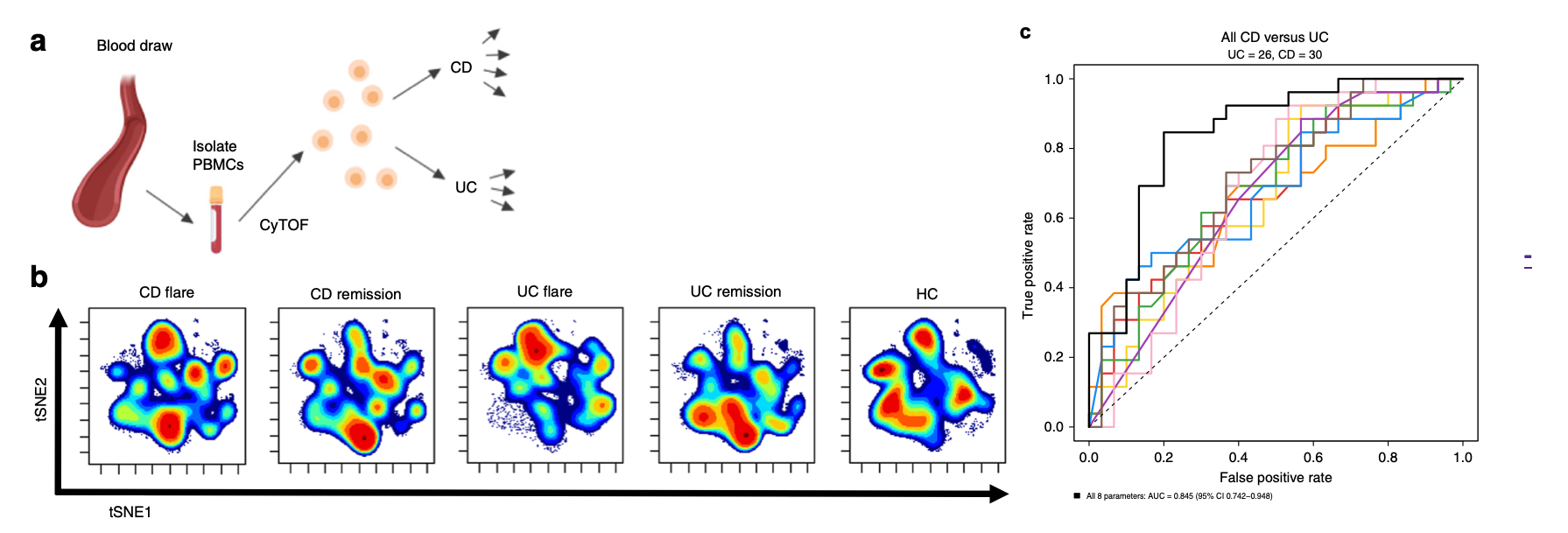
Figure description: Circulating leukocytes distinguish Crohn's disease (CD) and ulcerative colitis (UC). A) Schematic of the study conducted. Blood was drawn from study subjects and peripheral blood mononuclear cells (PBMCs) were isolated. B) Samples were analyzed by mass cytometry (CyTOF) or flow cytometry for independent validation. C) The discriminatory performance of blood markers was compared to standard-of-care colonoscopy, and diagnostic models were optimized. Adapted from Rubin et al., Nature Communications, 2019.
Stage of Development
The researchers have identified several blood-based signatures that differentiate CD from UC, and most importantly perform similarly to the standard-of-care endoscopy/colonoscopy for patients who have active disease – the most significant clinical unmet need. They have developed the diagnostic markers on a training patient cohort using mass cytometry (CyTOF) and validated the discriminatory performance on an independent validation patient cohort dataset using conventional clinical flow cytometry. They continue to study the markers on additional patients and optimize the diagnostic model to differentiate other clinically-relevant subtypes of IBD and predict therapeutic response to improve precision medicine in IBD and save patients and the healthcare system time, cost and adverse effects.
Applications
- Blood-based diagnosis and sub-typing of inflammatory bowel disease (IBD) patients
Advantages
- Method is less invasive, safer and cheaper than standard-of-care endoscopy/colonoscopy.
- Potentially more sensitive and specific than standard-of care endoscopy/colonoscopy, serology, and stool markers.
- Can be performed on flow cytometers commonly found in clinical diagnostic labs.
- Could be introduced clinically to reduce delay, risk, discomfort and cost to patients, and improve outcomes by arriving at a definitive diagnosis faster.
- Could be easily adapted as a companion diagnostic for existing or emerging therapies.
Publications
- Samuel J. S. Rubin, Lawrence Bai, Yeneneh Haileselassie, Gotzone Garay, Chohee Yun, Laren Becker, Sarah E. Streett, Sidhartha R. Sinha & Aida Habtezion, Mass cytometry reveals systemic and local immune signatures that distinguish inflammatory bowel diseases, Nature Communications 10, Article number: 2686 (published 19 June 2019).
- Gubatan J, Rubin SJS, Bai L, Haileselassie Y, Levitte S, Balabanis T, Patel A, Sharma A, Sinha SR, Habtezion A. "Vitamin D Is Associated with ?4?7+ Immunophenotypes and Predicts Vedolizumab Therapy Failure in Patients with Inflammatory Bowel Disease." J Crohns Colitis. 2021 Dec 18;15(12):1980-1990.
- Gottfried-Blackmore A, Rubin SJS, Bai L, Aluko S, Yang Y, Park W, Habtezion A. Effects of processing conditions on stability of immune analytes in human blood." Sci Rep. 2020 Oct 15;10(1):17328.
Related Links
Patents
- Published Application: WO2020142276
- Published Application: 20210396753
Similar Technologies
-
Fluorescent saccharide sensors for early detection of gastrointestinal diseases S17-396Fluorescent saccharide sensors for early detection of gastrointestinal diseases
-
Allergy testing with small whole blood samples S09-004Allergy testing with small whole blood samples
-
Biomarkers for Ovarian Cancer Diagnostics Prognostics as a Guide to Individualized Therapies S15-338Biomarkers for Ovarian Cancer Diagnostics Prognostics as a Guide to Individualized Therapies