Docket #: S17-233
RASER – a synthetic system for targeting therapy to tumors with hyperactive signaling
Researchers in Prof. Michael Lin's laboratory have developed a viral-based cancer therapy platform that could be used for targeting treatment to cancer cells with aberrant signaling in EGFR or HER2 pathways. A large fraction of solid tumors, especially breast, colorectal, head and neck, brain and lung cancers are driven by constitutively active signaling that promotes cell growth, proliferation or survival. Conventional anti-cancer drugs interfere with this signaling by directly interacting with one or more proteins in the pathway, acting on both oncogenic and healthy cells indiscriminately. This can lead to toxic side effects and drug resistance. To circumvent these issues, RASER (Rewiring of Aberrant Signaling to Effector Release) creates a synthetic pathway with a two-component system that uses cell signaling as a trigger rather than a target.
Briefly, RASER consists of a cytosolic component and a membrane-tethered component. Hyperactive EGFR or HER2 signaling induces interaction between the two RASER components, causing release of a cargo from the membrane-tethered component. The cargo can be customized to either kill the oncogenic cell or induce a transcriptional response. For effectors that are pro-apoptotic proteins, the net effect of the treatment is selective apoptosis in cells with oncogenic levels of EGFR or HER2 signaling. For effectors that are Cas9-based transcriptional activators, the net effect is upregulation of genes of choice, such as immunostimulatory genes, in cells with oncogenic levels of EGFR or HER2 signaling. The basic modular platform can be programmed and personalized to detect different signaling pathways with different sensor elements and/or induce a variety of outputs with different effector elements (e.g., direct induction of apoptosis, transcription of apoptosis-inducing genes, gene editing).
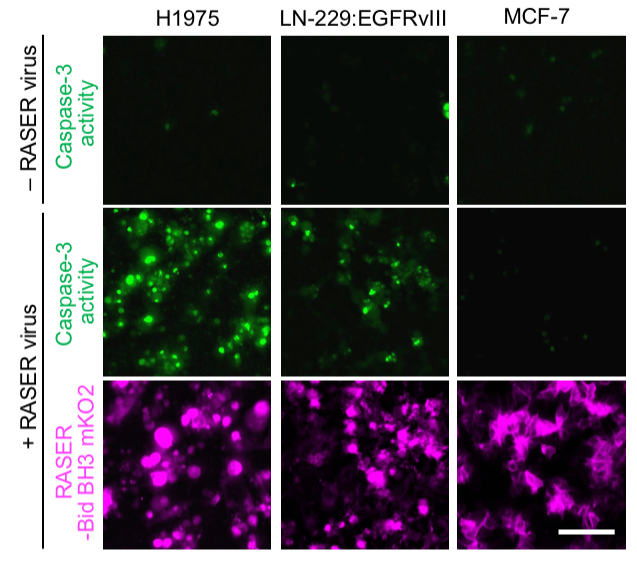
Figure legend: Killing of EGFR-mutant cancer cells by RASER virus infection. Top row, no virus. Middle row, caspase-3 reporter shows apoptosis in H1975 and LN-229:EGFRvIII, cancer cells with hyperactive EGFR, but not MCF-7 cells, which are not dependent on EGFR or HER2. Bottom row, direct fluorescence of orange fluorescent protein mKO2 fused to apoptotic cargo Bid BH3.
Stage of Research
The inventors have demonstrated proof-of-principle of the two-component ErbB-RASER system in vitro. In these studies, the sensing component was sensitive and specific to constitutive ErbB signal, activating a HCV NS3 protease to induce programmed responses from a range of effector components. The specific effector components tested were Bax (to activate BH3 pro-apoptotic proteins), FoxO (to activate pro-apoptotic transcription factors) and Cas9.
Applications
- Cancer therapeutics - viral-based therapy to target tumors with hyperactive ErbB-family receptor tyrosine kinases (EGFR, HER2, HER3, HER4)
Advantages
- Personalized, targeted delivery:
- RASER is designed so the therapeutic cargo is only released in cells with oncogenic levels of signaling, limiting impact on healthy cells
- therapeutic agent is mobilized by a sensing component that can be programmed based on the specific overexpression profile of a patient's tumor
- Decreased chance of resistance - RASER does not directly target proteins in a cell signaling pathway, therefore it avoids a common source of drug resistance from conventional therapies (i.e., mutations in the signaling protein that is being targeted by the drug)
- Generalized platform - modular components can be programmed to target overexpression from a range of tyrosine kinases
- Standard vectors - compact constructs for each of the two components designed to be packaged into standard viral vectors
Publications
- H.K. Chung, X. Zou, B.T. Bajar, V.R. Brand, Y. Huo, J.F. Alcudia, J.E. Ferrell Jr, M.Z. LIn A compact synthetic pathway rewires cancer signaling to therapeutic effector release Science 03 May 2019.
Related Links
Patents
- Published Application: 20190024070
- Published Application: 20190256833
- Issued: 11,939,609 (USA)
Similar Technologies
-
CRISPR/Cas9-mediated Genome Editing to Treat EGFR-mutant Lung Cancer S15-441CRISPR/Cas9-mediated Genome Editing to Treat EGFR-mutant Lung Cancer
-
CRISPR-Cas Based Treatment for Latent Viral Infections S14-222CRISPR-Cas Based Treatment for Latent Viral Infections
-
Small-molecule inhibitors of dynein motor proteins S10-155Small-molecule inhibitors of dynein motor proteins