Docket #: S12-094
Method and Apparatus for Sample Delivery in Serial Femtosecond X-ray Crystallography: Electrospinning Protein Crystals in Vacuo
Stanford researchers have developed a liquid microjet which provides the first nanoflow capability for serial femtosecond crystallography (SFX) with x-ray lasers.
SFX is an emerging method for 3D structure determination that extracts structural information from nano- to micron-sized crystals at room temperature. This method relies upon intense X-ray pulses that are sufficiently short to pass through the sample before the onset of significant radiation damage (diffraction-before-destruction). SFX therefore promises to break the correlation between sample size, damage and resolution in structural biology.
Precious protein sample consumption can be reduced by 60-100 times. A prototype has been demonstrated to provide 2 Angstrom protein structure in LCLS experiments while consuming 140 micrograms of protein in 50 microliters of sample volume.
Figures 1-3

Figure 1.Nanoflow electrospinning protein microcrystal suspensions in vacuo for serial femtosecond crystallography at the LCLS Coherent X-ray Imaging endstation. An electrospun microjet (a, scale bar 150 ?m) of a thermolysin crystal suspension, (microscope image, b),positioned 1 mm from the X-ray interaction point. An average of 2 mJ is delivered in each 40 fs pulse of 9.7 keV X-rays. Single pulse diffraction patterns from single crystals are recorded on a Cornell-SLAC Pixel Array Detector (CSPAD). A virtual powder pattern from 1024 LCLS shots that produced ?16 Bragg peaks each (c) shows diffraction beyond 4 Å. Purple and yellow squares denote the portion of CSPAD shown in the virtual powder pattern. From Sierra et al Acta Crystallographica D, 2012

Figure 2. Tuning the sample flow rate of electrospun microjets into the nanoflow regime using capillary ID and the pressure difference between the liquid reservoir and the vacuum chamber. The sample flow rate was measured for 30% w/v glycerol, 10% w/v PEG 2000, pH 6.5, 5 mM CaCl2, and 100mM MES buffer solution emitted into vacuum from 50, 75 and 100 ?m ID silica capillaries that were 114, 110, and 120 cm long, respectively. Linear fits were added to aid the eye. The range of flow rates for published SFX experiments is noted in red for comparison. From Sierra et al Acta Crystallographica D, 2012
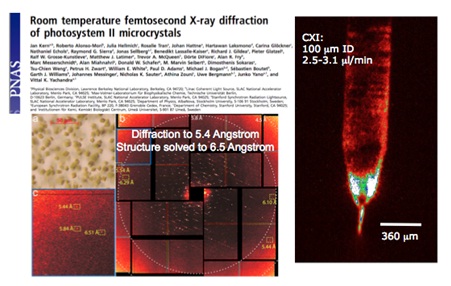
Figure 3. Room temperature diffraction from Photosystem II membrane protein crystals at LCLS. (a) protein crystals, (b) single-shot diffraction from single microcrystal, (c) high resolution Bragg spot detection, (d) microscope image of liquid microjet emitting from 100 micron inner diameter silica capillary. From Kern et al, PNAS 2012.
Stage of Research
Applications
- Structural biology and Drug Design - proteins that form small crystals can be analyzed by x-ray lasers using this nanoflow sample delivery method.
- General Practical Implications
- Lower sample consumption rate opens SFX to more precious samples
- Simple design allows for facile sample recovery
- Sample settling issue is resolved using highly viscous solutions
- Open access in the vacuum chamber facilitates complex experiments such as simultaneous x-ray emission spectroscopy/x-ray diffraction or time-resolved pump-probe experiments
- Government possibilities for use:
- SFX with 4th generation x-ray lasers like the LCLS and Next Generation Light Source
- SFX at 3rd generation synchrotrons
- Other single-shot x-ray diffraction experiments such as virus imaging, catalytic nanomaterials, or solution scattering.
- Sample delivery source for mass spectrometers
- Commercial possibilities for use:
- SFX with 4th generation x-ray lasers like the LCLS and Next Generation Light Source
- SFX at 3rd generation synchrotrons
- Other single-shot x-ray diffraction experiments such as virus imaging, catalytic nanomaterials, or solution scattering.
- Sample delivery source for mass spectrometers
Advantages
- Low cost, easy to use, simple design
- 60-100 times reduced sample consumption rate
- Open access to liquid jet
- Ability to probe variable jet thickness with x-rays
- Compatible with viscous samples
Publications
- "Nanoflow electrospinning serial femtosecond crystallography". Sierra, RG, Laksmono, H, Kern, J, Hattne, J, Alonso-Mori, R, Gloeckner, C, Tran, R, Hellmich, J, Lassalle-Kaiser, B, Schafer, DW, Sellberg, J, McQueen, TA, Fry, A, Messerschmidt, M, Miahnahri, A, Seibert, MM, Hampton, CY, Starodub, D, Loh, NTD, Zwart, PH, Milathianaki, D, White, WE, Adam, PD, Boutet, S, Williams, GJ, Messinger, J, Sauter, NK, Zouni, A, Bergmann, U, Yano, J, Yachandra, VK, & Bogan, MJ. Acta Crystallographica D; November 2012.
- "Room temperature femtosecond X-ray diffraction of photosystem II microcrystals". Jan Kern, Roberto Alonso-Mori, Julia Hellmich, Rosalie Tran, Johan Hattne, Hartawan Laksmono, Carina Glöckner, Nathaniel Echols, Raymond G. Sierra, Jonas Sellberg, Benedikt Lassalle-Kaiser, Richard J. Gildea, Pieter Glatzel, Ralf W. Grosse-Kunstleve, Matthew J. Latimer, Trevor A. McQueen, Dörte DiFiore, Alan R. Fry, Marc Messerschmidt, Alan Miahnahri, Donald W. Schafer, M. Marvin Seibert, Dimosthenis Sokaras, Tsu-Chien Weng, Petrus H. Zwart, William E. White, Paul D. Adams, Michael J. Bogan, Sébastien Boutet, Garth J. Williams, Johannes Messinger, Nicholas K. Sauter, Athina Zouni, Uwe Bergmann, Junko Yano, and Vittal K. Yachandra. Proceedings of the National Academy of Sciences; (2012) 109, 9721-9726
Related Links
Patents
- Published Application: 20130308756
Similar Technologies
-
Molecular vibrational spectroscopy method for early ovarian cancer detection S18-549Molecular vibrational spectroscopy method for early ovarian cancer detection
-
Nanoscale optical tomography with cathodoluminescence spectroscopy S14-086Nanoscale optical tomography with cathodoluminescence spectroscopy
-
Paperfuge: a low-cost, high-speed, human-powered centrifuge S15-444Paperfuge: a low-cost, high-speed, human-powered centrifuge