Docket #: S15-120
Alternative Energy with Efficient, Low-cost Mixing Entropy Battery
A team of Stanford engineers has developed an efficient battery that can convert salinity gradient power (a.k.a. “blue energy”) into electricity using low-cost, non-toxic electrode materials. This invention generates energy using the same principle as the mixing electron battery (“MEB”, described in Stanford Docket S10-123). However, it improves net power output because it employs low-cost electrode materials that do not require the initial energy investment of a charge step. This battery could be used to generate energy in any natural system that is alternately flushed with freshwater and saline water (e.g., desalination facilities, estuaries, or ships that move between ocean and freshwater environments). It has an energy potential roughly equivalent of the energy required to operate a wastewater treatment plant.
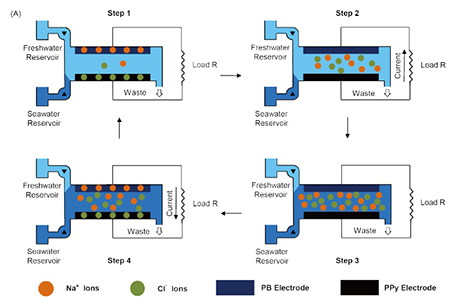
Schematic and typical cycle representing the working mechanism of the mixing entropy battery using a Prussian Blue (PB) cationic electrode and a polypyrrole (PPy) anionic electrode.
Stage of Research
The inventors have tested 3cm2 electrodes in an MEB and produced energy during both freshwater flushes and wastewater flushes while eliminating the charge step and upfront energy investment of previous MEBs. The MEB had over 93% capacity retention over 50 cycles of operation. Furthermore, the inventors have built a prototype of a larger scale (10 cm2) electrode.
Applications
- Alternative energy from wastewater - can recover energy from water treatment or desalination facilities wherever saltwater and freshwater are both present
Advantages
- High net energy:
- no power investment needed during the charge step
- only operation needed is alternative flushing of freshwater and seawater
- voltage ratios of 85.8% and 95.4%, and over 93% capacity retention over 50 cycles of operation
- overall energy potential ~0.6 kWh/m3 of treated wastewater (roughly equivalent to energy required to operate a wastewater treatment plant)
- Low cost materials - anode ($3/kg) and cathode ($1/kg) materials are significantly less expensive than silver and manganese oxide electrodes used in original MEB design
- No membranes or complex electronics
Publications
- Ye M. et al. Performance of a mixing entropy battery alternately flushed with wastewater effluent and seawater for recovery of salinity-gradient energy. Energy & Environmental Science. 10 April 2014 DOI: 10.1039/c4ee01034e
Related Links
Patents
- Issued: 10,003,110 (USA)
Similar Technologies
-
Nighttime Electrical Power Generation via Radiative Cooling S20-222Nighttime Electrical Power Generation via Radiative Cooling
-
Mixing Entropy Battery S10-123Mixing Entropy Battery
-
Flow Batteries with High Energy Density Redox-active eutectic liquid S18-551Flow Batteries with High Energy Density Redox-active eutectic liquid