Docket #: S25-283
Automated Artery-Vein Separation on Brain CTA for Enhanced Stroke Prognosis and Collateral Evaluation
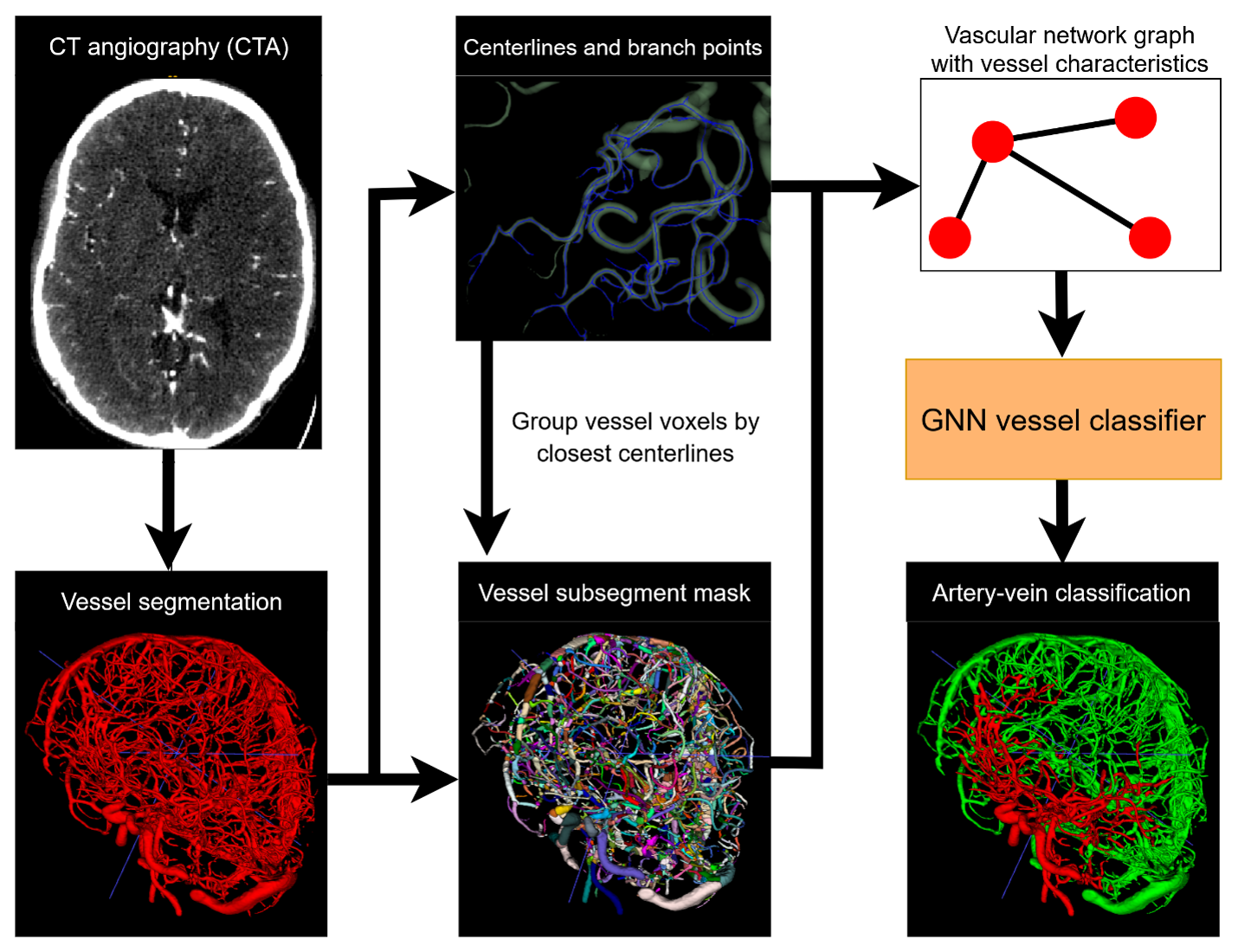
Stanford researchers have developed a technology for the automated separation of arteries and veins in single-phase brain CT angiography (CTA) using graph neural networks, enabling precise collateral scoring and improved stroke prognosis. This innovation enhances diagnostic accuracy and supports therapeutic research in cerebrovascular diseases.
In neurovascular imaging, distinguishing between arteries and veins on single-phase brain CT angiography is challenging due to similar contrast opacification, making accurate diagnosis and prognosis difficult. Traditional methods rely on time-consuming manual tracing by neuroradiologists, which is impractical in acute settings and suffers from interrater variability. Existing automated techniques using conventional deep learning struggle with this task, and alternative methods like CT perfusion imaging are rarely available and costly.
This technology addresses these issues by leveraging graph neural networks to automate artery-vein separation, enhancing diagnostic precision, and enabling quantitative collateral evaluation for better stroke outcome prediction. This innovation addresses the major problem of distinguishing between arteries and veins. By constructing vascular network graphs and applying graph neural networks, the technology enables precise and automated artery-vein classification, significantly improving diagnostic accuracy and speed. The technology demonstrated high classification performance that enhances stroke prognosis and supports therapeutic research in cerebrovascular diseases.
Stage of Development
Prototype
Figure
Applications
- Automated occlusion detection in acute ischemic stroke and cerebral venous thrombosis
- Quantitative evaluation of collateral circulation for stroke prognosis
- Drug and device development for improving cerebrovascular flow
Advantages
- Improved diagnostic accuracy and speed for neurovascular diseases
- Enhanced prognostic precision for ischemic stroke outcomes
- Reduced interrater variability compared to expert-based evaluation methods
- Comprehensive evaluation of arterial and venous circulation for therapeutic research
- More detailed diagnostics and automatic extraction of anatomical structures enabled by techniques used in this method
Related Links
Similar Technologies
-
Convex Formulation of Continuous Time Heartbeat Dynamics S23-288Convex Formulation of Continuous Time Heartbeat Dynamics
-
Imaging software for task-evoked conditions S18-083Imaging software for task-evoked conditions
-
Automated Gait Analysis using video S18-010Automated Gait Analysis using video