Docket #: S23-293
On-chip laser microdissection for single cell sorting
Stanford researchers have developed a new on-chip system for laser microdissection that enables more reliable isolation of single cells or small regions of tissue and permits long-term sample storage.
Laser microdissection enables scientists to precisely visualize specific cells or small regions of tissue under a microscope and isolate them for downstream analysis. This is especially critical when analyzing specific cell types in heterogenous samples like tumors. However, available instruments for laser microdissection have significant drawbacks, as they are unable to reliably isolate single cells, are error-prone, and struggle with physical influences such as electrostatics. Additionally, such open systems leave samples prone to contamination and cannot reliably store samples for later analysis.
Stanford researchers therefore developed an improved system combining laser microdissection with microfluidics. Cells are isolated using a laser and dispensed into buffer running through a microfluidic chip, where a system of valves sorts different samples. In contrast to systems that rely on gravity, this allows for reliable and standardized sample isolation. This also protects samples from contamination and enables their long-term storage. Additionally, a miniaturized format allows for cheap and easy integration into upstream imaging and downstream analysis workflows.
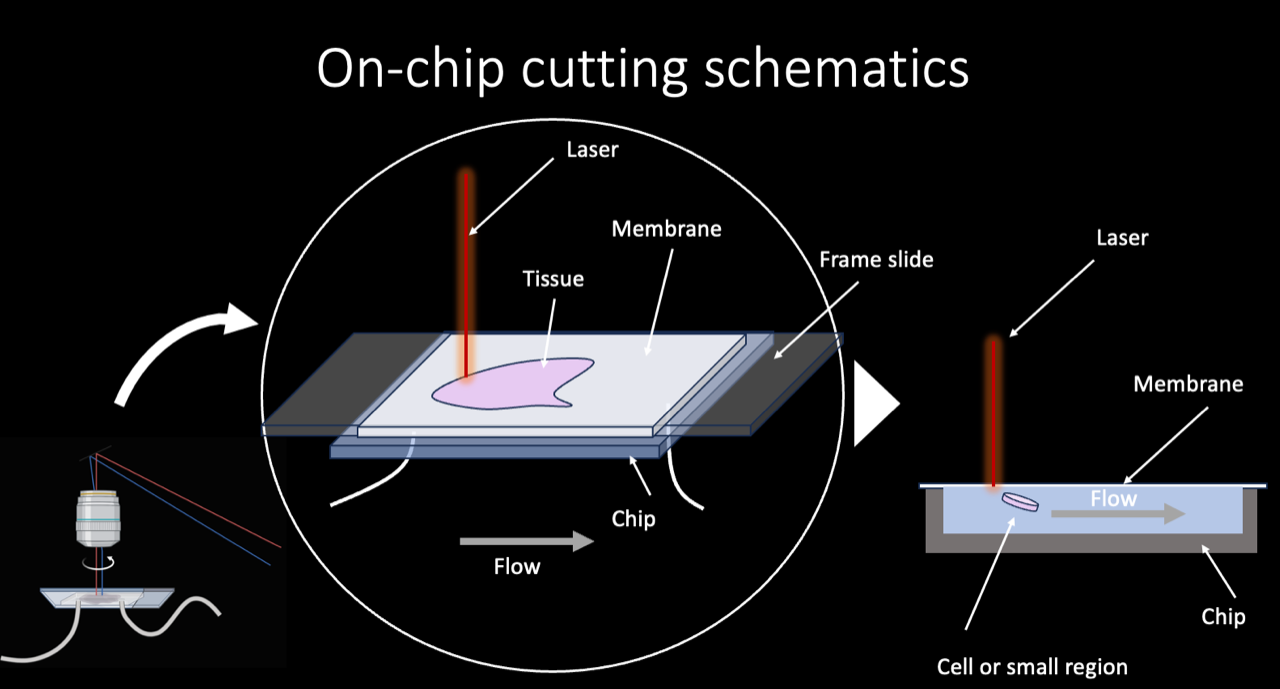
Stage of Development
Prototype: Reserachers have demonstrated isolation of cell shapes from a sample and their transportation though the microfluidic chip
Applications
- Precise cell isolation and analysis for basic biology research
- Single-cell genomics, proteomics, and transcriptomics
- Single cell analysis for drug development
- Analysis of patient samples for diagnostics and biomarker discovery
- Label-free enrichment of rare cell populations
- Generation of monoclonal colonies
Advantages
- Reliable and standardized sample collection
- Closed system protects sample from contamination and permits easy sample storage
- Can be automated
- Can integrate with upstream tissue processing and downstream analysis platforms
- Miniaturization permits inexpensive integration with other imaging platforms
Related Links
Patents
- Published Application: WO2026015754
Similar Technologies
-
MONTAGE: Unlocking Spatial Biology to Analyze Tumour Microenvironment S25-060MONTAGE: Unlocking Spatial Biology to Analyze Tumour Microenvironment
-
QUANTIFICATION OF CELLULAR PROTEINS USING BARCODED BINDING MOIETIES S20-449QUANTIFICATION OF CELLULAR PROTEINS USING BARCODED BINDING MOIETIES
-
Tetrapod Phase Mask Microscopy S15-038Tetrapod Phase Mask Microscopy