Docket #: S18-498
Combination of differentiation therapy and metabolic intervention to treat neuroblastoma
Researchers at Stanford have developed a combination therapy to treat neuroblastoma, the most common and deadly solid tumor in childhood. Neuroblastoma derives from neural crest cells that fail to exit the cell cycle and differentiate. Retinoic acid (RA) has been used to treat this cancer as it leads to cell cycle arrest and induces differentiation. However, neuroblastoma is often resistant to this therapy. Hypoxia, a common metabolic stress existing in the tumor microenvironment, promotes dedifferentiation of neuroblastoma cells and increases resistance to RA. Thus, new methods of treating neuroblastoma are needed.
To help meet this need the inventors have identified mechanisms underlying hypoxia-mediated therapy resistance and developed a new approach to improve the response to RA-based differentiation therapy. Here, the inventors provide a combination therapy using both RA and acetate supplementation. This combination provides metabolic interventions which induce differentiation of cancer cells.
This technology provides an effective therapeutic approach to increase the sensitivity of the hypoxic tumor to differentiation therapy and thus treat neuroblastoma. Further, in addition to neuroblastoma, RA and other retinoids have shown promising anti-cancer effects in cell lines or preclinical models of other types of solid tumors, it is expected that this combination therapeutic strategy might have a broad application that is not limited to neuroblastoma treatment.
Stage of research
Initial validation studies show great promise. Additional development is ongoing.
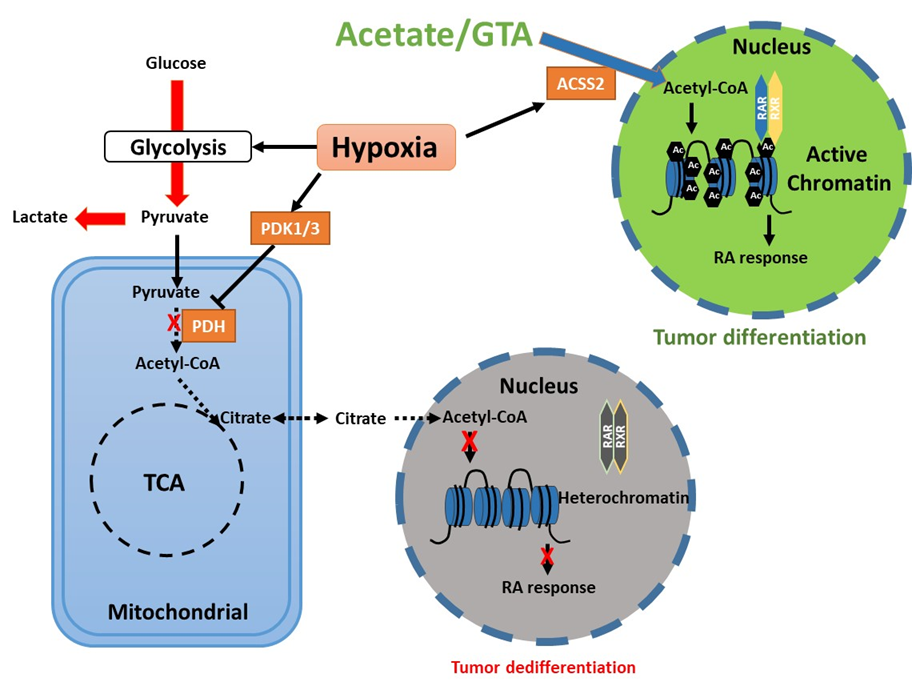
Hypoxia is a common tumor microenvironmental stress that represses the differentiation induced by retinoic acid (RA) by reducing global histone acetylation and chromatin accessibility. Upon hypoxia, pyruvate dehydrogenase kinases (PDKs) are induced to phosphorylate pyruvate dehydrogenase (PDH), thereby blocking pyruvate entry into the TCA cycle, reducing acetyl-CoA and citrate generation, and promoting the Warburg effect. Acetate supplementation restores chromatin accessibility along with differentiation markers expression and neuron differentiation. These findings suggest that combining differentiation therapy in the form of RA with acetate supplementation might represent an effective therapeutic strategy for cancer treatment.
Applications
- Treatment for neuroblastom
Advantages
- Improve the response of the hypoxic tumor to differentiation therapy
- More effective therapeutic strategy to treat neuroblastoma
- Can be used in combination with conventional treatments
Publications
- Li et al. Cell Death and Disease (2020) Acetate supplementation restores chromatin accessibility and promotes tumor cell differentiation under hypoxia
Related Links
Patents
- Published Application: 20200261393
- Issued: 11,684,601 (USA)
Similar Technologies
-
Therapeutic targets for glioma tumors S14-408Therapeutic targets for glioma tumors
-
Methods to improve phagocytosis for treatment of age-related diseases S17-443Methods to improve phagocytosis for treatment of age-related diseases
-
Therapeutic targets to limit high-grade glioma spread S17-248Therapeutic targets to limit high-grade glioma spread