Docket #: S15-240
Controlling AAV receptor expression to improve testing and validation of AAV gene therapy products
Adeno-associated virus (AAV) vectored products are currently leading candidates for gene therapy applications with multiple approved products and many more in clinical trials. While AAV has been successfully used for gene delivery for many years, the receptor for this virus was only recently discovered (Pillay, 2016). The multi-serotype AAV receptor (AAVR) has been shown to be critical for transduction both in vitro and in vivo for most AAV serotypes including the major serotypes used in the clinic. Overexpression of AAVR has also been shown to increase transduction in vitro for multiple AAV serotypes that are known to poorly transduce cells. This finding has allowed for the development of cells that can be used to functionally titrate AAV serotypes where it was previously difficult or impossible.
Current problems
- There is no way to functionally titrate many clinically relevant AAV serotypes.
- Quality control for drug products is limited to genome quantification and capsid quality assay.
- Testing AAV gene therapies in mice can be limited by AAV receptor expression.
Applications
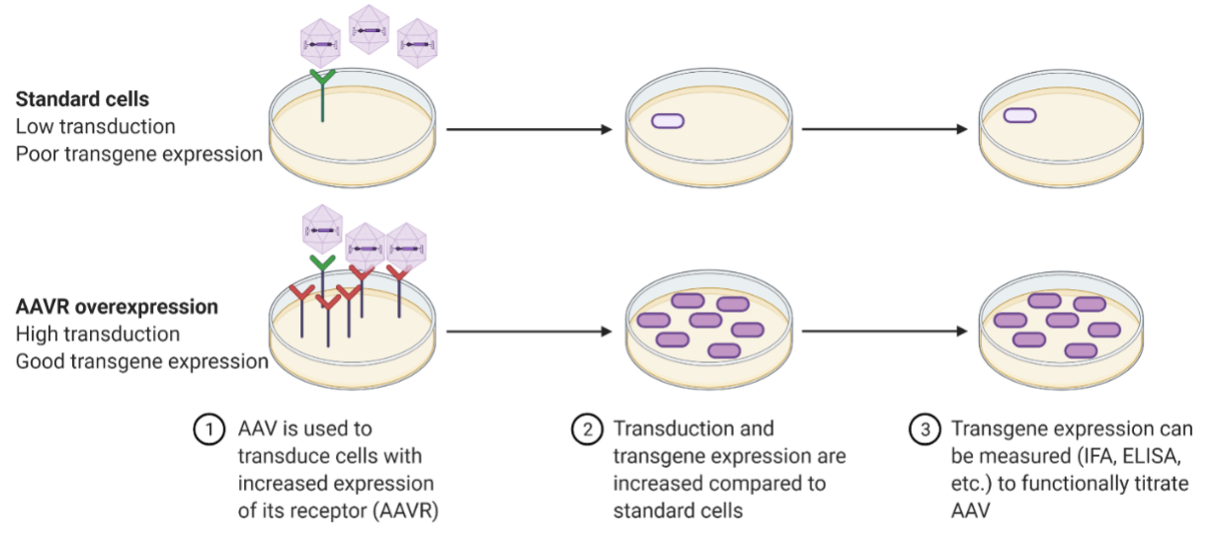
Quality control of AAV production for clinical serotypes by functional titration
- Many clinical serotypes poorly transduce cell lines in vitro. AAVR overexpression allows for high levels of transduction in vitro by multiple clinically relevant AAV serotypes including AAV8 and AAV9.
- The AAVR overexpressing cell lines can have >1000-fold increases in transduction compared to wild-type cells.
- Allows for a functional in vitro testing of AAV for preclinical research and drug product validation.
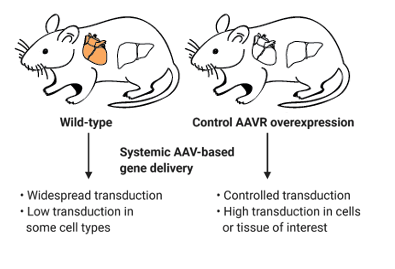
Mouse model development for preclinical research for AAV-based gene therapy
- Regulated AAVR expression allows for targeted AAV-vectored gene delivery in mouse models.
- AAVR overexpression allows for transduction of poorly transduced cell types in vivo.
- Mice will allow for preclinical model development and testing of AAV-gene therapy products in vivo
Publications
- Pillay, S., N. L. Meyer, A. S. Puschnik, O. Davulcu, J. Diep, Y. Ishikawa, L. T. Jae, J. E. Wosen, C. M. Nagamine, M. S. Chapman, and J. E. Carette. "An Essential Receptor for Adeno-associated Virus Infection.' Nature 530.7588 (2016): 108-12.
Related Links
Patents
- Published Application: WO2017083423
- Published Application: 20180327752
- Issued: 10,633,662 (USA)
Similar Technologies
-
Efficient homologous recombination of large transgenes using AAV donor vectors S16-453Efficient homologous recombination of large transgenes using AAV donor vectors
-
Robust factor IX minigene expression cassette (TTR) S06-099Robust factor IX minigene expression cassette (TTR)
-
Therapeutic to restore vision loss S18-081Therapeutic to restore vision loss