Docket #: S22-133
Enrichment of clinically relevant cell types using receptors
Introduction: Blood cell transfusion plays a vital role in modern medicine–supporting surgery, obstetrics, trauma care, and cancer chemotherapy. In the US alone, more than 12 million red-cell units are consumed annually. However, the availability is contingent on donor material, resulting in supply constraints and safety concerns. Globally, shortages pose a significant healthcare challenge, expected to worsen with aging populations and diminishing donor numbers. To address these growing medical needs, ex vivo manufacture of red blood cells (RBCs) from induced pluripotent stem cell (iPSC) producer lines emerges as a renewable and scalable solution that offer potential benefits compared to donor blood, including a lower risk of infectious disease transmission, streamlined production, product uniformity, and ability to source or genetically engineer antigen-negative cells. However, this approach remains extremely costly, owing in large part to purified, clinical-grade recombinant cytokines required to stimulate producer cells for expansion and differentiation into erythroid cells. Because erythropoietin (EPO) signaling through the EPO receptor (EPOR) is indispensable to RBC development, of all components in erythroid-promoting media, EPO is by far the most costly.
Methods: We used the latest synthetic biology tools and genome engineering technologies to de-couple EPOR signaling from the EPO cytokine. Specifically, we devised a series of FKBP-EPOR chimeras that exchanged the requirement for native EPO ligand with an exogenous, orthogonal rapalog small molecule (SM) which we termed inducible EPORs (iEPORs). These were site-specifically inserted in vitro into primary human hematopoietic cells (HSCs) via CRISPR/AAV-mediated genome editing at various HSC and RBC-relevant loci (CCR5 with the PGK and SFFV promoters, EPOR, and HBA1). Using flow cytometry and ddPCR-based assays, we determined the fidelity (i.e., responsivity to SM) and potency (ability to drive differentiation from a stem cell state towards the erythroid lineage) of various iEPOR constructs (different signal peptides and extracellular and intracellular domain modifications) as well as the impact of inserting these chimeras at the aforementioned loci.
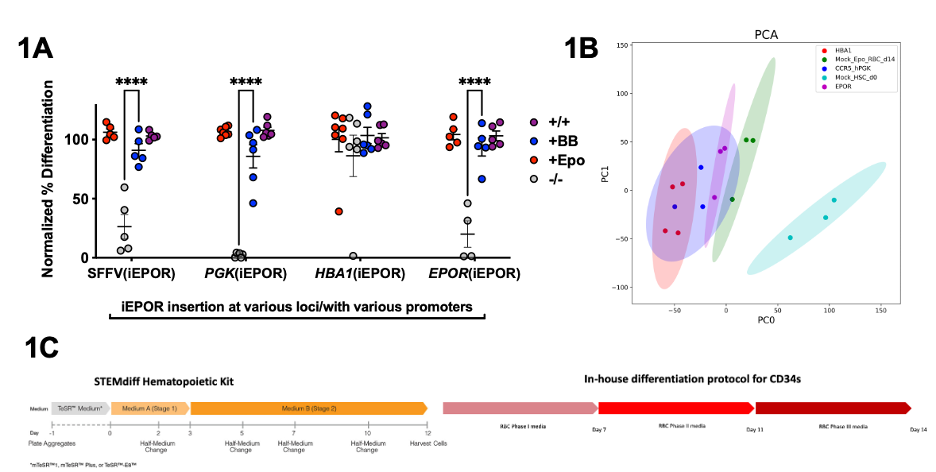
Results & Discussion: We first optimized iEPOR constructs through a highly effective signal peptide present on IL6 and inclusion of the naturally occurring intracellular domain truncation known to enhance erythropoiesis. Together, these modifications allowed us to drive HSCs to 100% differentiation into RBCs in the presence of the iEPOR-dimerizing small molecule. However, we also observed 26.2% differentiation in the absence of any endogenous or exogenous erythroid-biasing ligand. To increase the fidelity of our iEPOR, we integrated our iEPOR at the CCR5 locus and drove expression using the PGK promoter to ablate ligand-independent differentiation (Figure 1A). This identical HSC response to iEPOR in the presence of our small molecule versus native HSC in the presence of EPO was corroborated not only by flow cytometry-based markers of erythroid differentiation but also via HPLC-based assessment of hemoglobin A and F production. iEPOR-integrated HSCs differentiated as described clustered with natively differentiated HSCs vs undifferentiated HSCs by PCA covariance plots and differential gene expression heatmaps (Figure 1B). We observed effectively identical counts of hemoglobin RNA supporting our HPLC findings. Finally, we integrated our optimized iEPOR construct into an iPSC line and devised a consolidated, differentiation strategy from induced pluripotent stem cells to HSCs and, ultimately, RBCs (Figure 1C). Together, this proves our approach serves an ex vivo, EPO-independent RBC production strategy that closely mimics native EPOR signaling by surface biomarkers, relevant protein output, and transcriptional profile. By our calculation, 1nM of dimerizer is approximately 500-fold less costly than 3U/mL of EPO cytokine. This would therefore reduce the cost of EPO cytokine required to generate a single unit of RBCs from $2,760 to approximately $6. Given that dependence on costly cytokines is a common barrier to production of cell-based therapeutics, in the future we believe the strategies defined in this work may enable scalable and renewable ex vivo production of a wide variety of clinically relevant cell types including platelets, neutrophils, T cells.
Stage of Development
Research – in vivo
Related Technologies
S21-196: Differential Proliferation of Human HSPCs Using Truncated Erythropoietin Receptors
S21-175: Potential Curative Treatment for Alpha-Thalassemia Using CRISPR-Mediated Genome Editing
Applications
- Hemoglobinopathies
Advantages
- Greater efficacy by inducing bias in transplanted HSC to yield higher RBC production.
- Lower toxic and mutagenic regimens are required to stimulate high RBC production.
- Easy integration into current treatment workflow.
Related Links
Patents
- Published Application: WO2024086518
Similar Technologies
-
Human helicase-based system for enhanced gene integration S24-152Human helicase-based system for enhanced gene integration
-
Using gene therapy and metabolite supplementation to treat ciliopathies S23-278Using gene therapy and metabolite supplementation to treat ciliopathies
-
Dual-Agent Conditioning Improves Hematopoietic Stem Cell Engraftment S20-361Dual-Agent Conditioning Improves Hematopoietic Stem Cell Engraftment