Docket #: S20-315
Hematopoietic cell chimerism and transplantation tolerance
Researchers at Stanford developed a novel technique to induce persistent mixed hematopoietic cell chimerism in organ recipients to protect against organ graft rejection and increase immune tolerance. Over 40,000 organ transplants are performed annually in the US, with over 95% of organs coming from HLA mismatched living or deceased donors. Organ recipients depend on immune suppression (IS) medication for the rest of their lives to prevent organ graft loss and immune mediated rejection, both of which leads to poor patient survival. Adverse IS-induced side effects coupled with a rigorous lifelong medication regime necessitates alternative techniques to traditional IS medication.
The inventors developed a revolutionary technique that leverages mismatched hematopoietic cells from living and deceased donors, eliminating the need for long-term IS medication and increasing patient survival rates post-organ transplant. Patients receiving an organ transplant will be administered a mixed dose of T-cells or hematopoietic stem cells from donors to induce persistent immune tolerance. The treatment leverages non-physiologic ratios of donor cells from either living or decease donors. As decease donors make up the largest pool of organ donors, the treatment provides a novel solution to create host conditioning for organ recipients. Prior clinical trials showcased the safety and efficacy of the invention, and additional clinical trials are currently underway at Stanford to demonstrate the proof-of-concept of the technique.
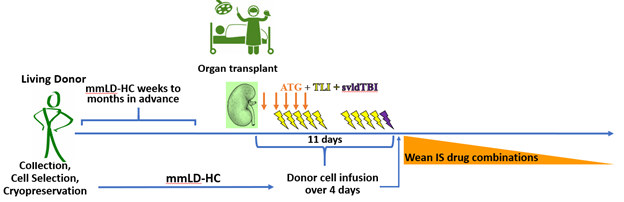
Timeline of treatment application from organ donor to post-organ transplant.
Stage of Development: Phase I clinical trial
Applications
- Organ preservation for transport and transplant
- Organ transplant
- Immune suppression
Advantages
- Applicable to any organs and tissue transplant
- Low medication dosages and short-term treatment
- Eliminates lifelong IS medication
Publications
- Busque, S., et al. Mixed chimerism and acceptance of kidney transplants after immunosuppressive drug withdrawal" Science Translational Medicine 12(528) Jan. 29, 2020.
Related Links
Patents
- Published Application: WO2022072320
- Published Application: 20240024362
Similar Technologies
-
Knock-In of Large DNA for Stable and Long-Term High Genomic Expression S20-259Knock-In of Large DNA for Stable and Long-Term High Genomic Expression
-
Engineered proteins to enhance the sensitivity of immune cells to IL-2 S17-190Engineered proteins to enhance the sensitivity of immune cells to IL-2
-
Coupling of Excitation and Neurogenesis in Neural Stem/Progenitor Cells S01-094Coupling of Excitation and Neurogenesis in Neural Stem/Progenitor Cells