Docket #: S13-327
Materials for capture, release, and separation of iodine and other halogens
Researchers in Prof. Hemamala Karunadasa's laboratory have developed inexpensive, robust, high capacity hybrid materials for reversible or irreversible capture of halogens (chlorine, bromine, and iodine gas). These non-porous organic-inorganic layered solids have high gravimetric and volumetric storage capacity. Further, they can be tuned for timed release to enable transportation of the halogen and to easily regenerate the capture material. They can also purify gases by separating different halogens. Iodine capture applications of this technology include sequestration and transportation of radioactive iodine from nuclear fuel streams; chemical purification; and solid-state antimicrobial materials for disinfection of surfaces and environment (for example, in air filters).
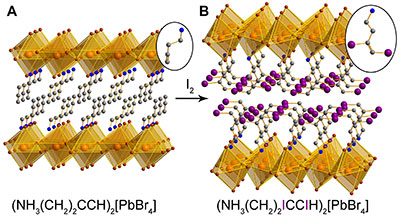
X-ray structures of iodine capture material: a before capture and b its reaction product with iodine. Insets show the individual cations in the organic layers. (Orange, purple, brown, blue, and grey spheres represent Pb, I, Br, N, and C atoms, respectively.)
Stage of Research
The inventors have created the materials as microcrystalline powder and oriented thin films. These materials have demonstrated gravimetric capacities up to 43% for iodine capture; tunable half-life for iodine release; and resistance to HCl, water, and NOx gases. The inventors continue to explore synthesis of nanoparticles and coating filters.
Applications
- Nuclear waste remediation - capture of radioactive iodine vapor in nuclear fuel streams in solid form
- Disinfection - timed release iodine to disinfect air streams (using a solid filter) or surfaces
- Iodine purification - materials can remove small amounts of bromine and chlorine gas to produce high-purity iodine gas
Advantages
- High capture capacity - high gravimetric and volumetric capacities for iodine vapor capture:
- materials have gravimetric capacities up to 43% (compared to ~23% for zeolites and 64 for metal-organic frameworks developed for iodine capture).
- non-porous materials with higher volumetric capacities than porous zeolites and metal-organic frameworks which is more relevant for long-term stationary storage
- Timed release - in the case of materials with reversible capture:
- enables inexpensive regeneration of material
- allows for short term radioisotopes to decay before relocation and transmutation
- half-life of iodine-release materials can be tuned from 3 hours to 3 days and may be further tuned (increase of two orders of magnitude from solution-state capture)
- Robust - resists HCl, high humidity, temperatures up to 170°C, and NOx gases
- Low cost fabrication - inexpensive solution-based bulk manufacturing at ambient temperature and pressure
- Solid state - membrane or powder-based release material can be used for iodine release in medical and disinfecting applications where solid release is preferable to liquid (e.g. air filters)
- Radiation attenuation - radioactive iodine is confined between lead-bromide sheets that attenuate beta, gamma and x-ray emission
- Selective - some materials can select for one halogen over another (e.g. for purification applications)
Publications
- "Reversible and irreversible chemisorption in nonporous-crystalline hybrids" Solis-Ibarra, D. and Karunadasa, H. I. Angew. Chem., Int. Ed. 2014, 53, 1039.
Related Links
Patents
- Published Application: WO2015035216
- Published Application: 20160193566
- Issued: 10,272,384 (USA)
Similar Technologies
-
Customizable, Porous Tissue Engineering Scaffold for 3D Cell Proliferation S12-089Customizable, Porous Tissue Engineering Scaffold for 3D Cell Proliferation
-
Self-Powered Electronic Skin S14-211Self-Powered Electronic Skin
-
Collagen Materials, Films and Methods S08-229Collagen Materials, Films and Methods