Docket #: S09-344
Monoclonal Antibodies to Identify and Remove Teratoma-initiating Stem Cells
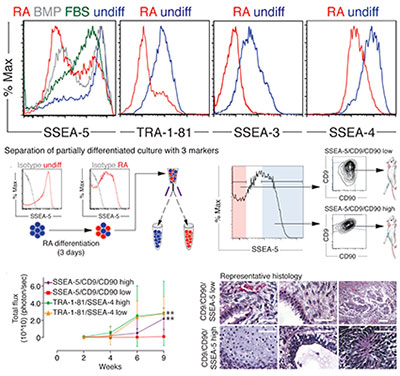
Researchers in Prof. Irving Weissman's lab have developed and patented antibodies and methods to prevent the formation of teratomas from human pluripotent stem cells used for regenerative medicine, cell therapy or research. When derivatives of embryonic stem cells or induced pluripotent stem cells (ESCs and iPSCs) are used as a source of therapeutics, one critical safety concern is that teratomas may form from residual undifferentiated pluripotent cells. This technology includes novel cell surface markers of pluripotency (including stage-specific embryonic antigen-5, or SSEA-5) and monoclonal antibodies to the markers which can be used to identify and remove these residual teratogenic cells non-invasively, prior to the transplantation of ESC or iPSC derivatives. The differentiated cell populations produced after pluripotent cell removal could be used for tissue regeneration, drug screening, or basic research.
Stage of Research:
The inventors have used monoclonal antibodies to SSEA-5 and two additional pluripotency markers for fluorescence-activated cell sorting (FACS), magnetic-activated cell sorting (MACS), immunopanning, or antibody-based separation methods to completely remove teratoma-formation potential from incompletely differentiated human ESC cultures.
Applications
- Regenerative medicine :
- removal of residual undifferentiated human ESCs/iPSCs prior to transplantation to prevent teratoma formation
- screen iPSC colonies to detect those that are full undifferentiated
- Research:
- detection, labeling and isolation of undifferentiated human ESCs and iPSCs, teratoma stem cells, pluripotent cells in vivo, in fixed tumors, and embryoid bodies to study ESC differentiation, iPSC line derivation and possibly cancer biology
- detection and screening of fully differentiated iPSC colonies and tracking the iPSC derivation process
Advantages
- Comprehensive, prospective removal of tumor-forming cells
- Universal protocols - the cell surface markers, antibodies and immunodepletion techniques can be used for any human pluripotent cell type, including patient iPSC lines
- No cellular modifications - cell surface markers are intrinsic to all cells, compared to chemical or genetic approaches which are more invasive and require modifications of the cells that will be transplanted
- Specific - the SSEA-5 antibody is highly specific for a glycan marker H-type 1 antigen on undifferentiated cells
- Versatile - the SSEA-5 antibody can be used at multiple stages in the production of an ESC/iPSC derived product (for example: early within a differentiation process to remove undifferentiated cells before allowing the product to fully mature and expand in number; as a screening tool to ensure that batches are devoid of pluripotent cells; and as a final phase in purification)
Publications
- Tang et al. An antibody against SSEA-5 glycan on human pluripotent stem cells enables removal of teratoma-forming cells. Nature Biotechnology. Aug 14, 2011.
- Krista Conger. Discovery may eliminate potentially lethal side effect of stem cell therapy. Inside Stanford Medicine. Aug 14, 2011.
Patents
- Published Application: WO2011094538
- Published Application: 20130028909
- Issued: 9,175,079 (USA)
Similar Technologies
-
Rapid iPS Cells from Adult Human Adipose Stem Cells S08-438Rapid iPS Cells from Adult Human Adipose Stem Cells
-
CytoTrace2: Methods and Systems for Determining Phenotypic States from Genomic Data with Interpretable AI S24-057CytoTrace2: Methods and Systems for Determining Phenotypic States from Genomic Data with Interpretable AI
-
Using Minicircle DNAs to Generate Viral-Free Induced Pluripotent Cells S09-309Using Minicircle DNAs to Generate Viral-Free Induced Pluripotent Cells