Docket #: S19-110
Next Generation Therapy for Heart Failure: Mediated Mitochondrial Transplantation
Researchers at Stanford are advancing a new treatment for heart failure based on the transfer of mitochondria-rich extracellular vesicles from iPSC-derived cardiomyocytes. Heart failure is the leading cause of hospital admission in the U.S. and the 5-year survival is still a dismal 50%. Key to heart failure is the imbalance between energy supply and demand. Current therapeutics attempt to correct this imbalance by reducing cardiac workload, but they do not target the primary energy source of the failing heart. Needed is an innovative therapy for cardiac disorders that targets intracellular bioenergetics directly. The new approach is based on the researchers' discovery that the induced cardiomyocytes (iCM) released mitochondria-positive extracellular vesicles (EV) that improved mitochondrial function and even restored the bioenergetics of recipient cardiomyocytes. This approach could solve many of the challenges of mitochondria transplantation and advance the current therapeutic regimen for patients.
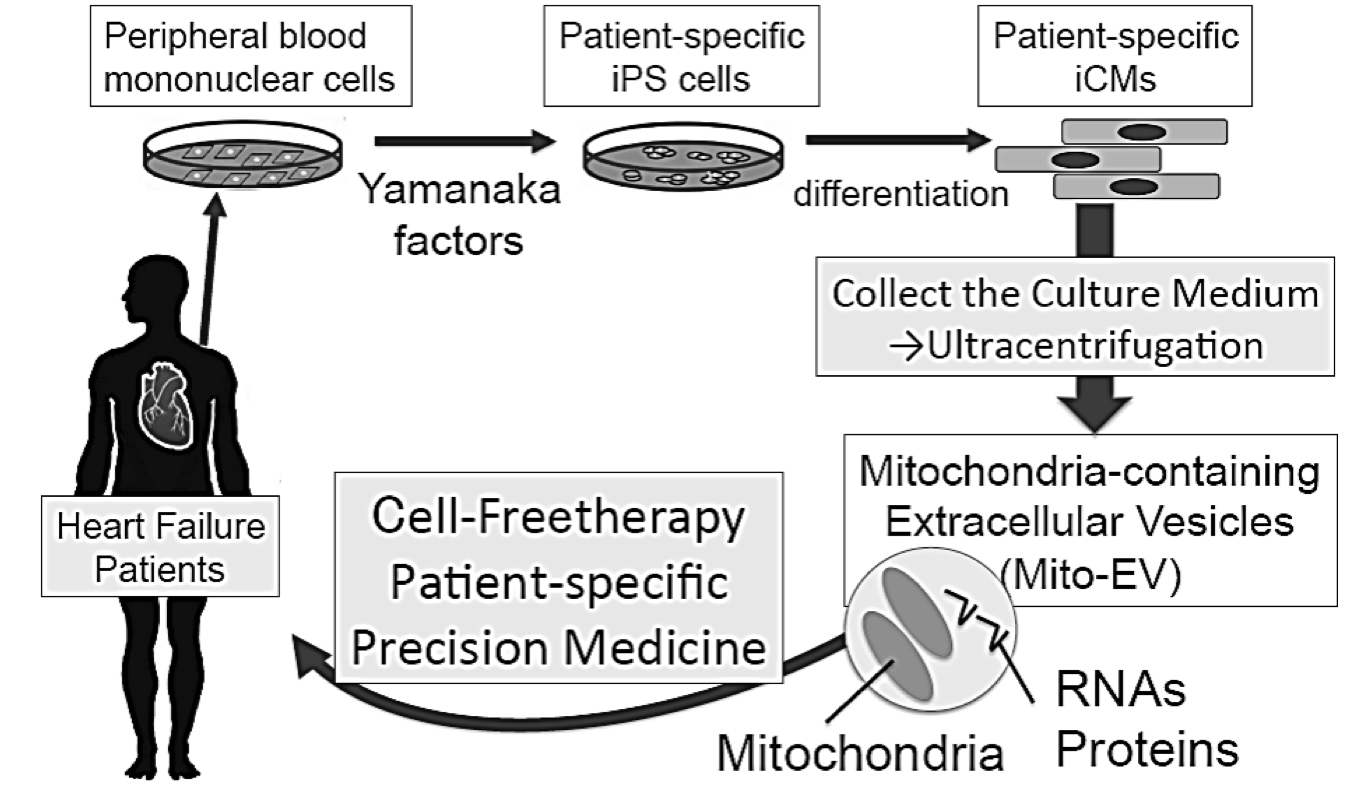
(schematic) Treating heart failure by transferring mitochondria derived from the patient's own stem cells back into the cells of the damaged heart muscle
Stage of Development
Intra-myocardial injection of mitochondria-rich EVs enhanced survival of hypoxia-injured iCMs in vitro and improved ventricular function and attenuated cardiac remodeling of ischemia-injured murine myocardium in vivo.
Applications
- Therapeutic for bioenergetic imbalance in heart failure
- Treatment for hypertrophic, arrythymogenic right ventricular, dilated, restrictive, and ischemic cardiomyopathy
Advantages
- Currently, clinical use of isolated mitochondria to treat heart failure is extremely challenging
- EV-mediated transfer of mitochondria can address key challenges
- Mitochondria inside EVs are protective, preventing calcium overload when compared to isolated mitochondria
- The lipid bilayer structure of the EVs prevents calcium ions from diffusing into the vesicles
- EVs are internalized by recipient cells through rapid fusion with cell membrane, resulting in direct release of the mitochondrial content into the cytoplasm
- The EVs facilitate quick transfer of EVs-mitochondria into recipient iCM. By contrast, isolated mitochondria were rarely internalized by recipient iCM within 24 hours.
- The transferred mitochondria integrated with host mitochondria and increased ATP level within three hours
Publications
- Santoso, M. R. et al. Exosomes From Induced Pluripotent Stem Cell-Derived Cardiomyocytes Promote Autophagy for Myocardial Repair JAHA 2020; 9(6):e014345
Related Links
Patents
- Published Application: WO2020232301
- Published Application: 20220233604