Docket #: S23-249
Use of recombinant osteopontin (SPP1) protein for prevention of foreign body response
Researchers at Stanford University have found that recombinant osteopontin (SPP1) protein reduces foreign body response (FBR) and thereby facilitates successful integration and function of implantable devices.
FBR is an immune response in which detected foreign material is encapsulated in dense fibrotic scar tissue. While this response is crucial for protecting the body from bioactive foreign substances, it can be a problem when implants are intentionally placed in the body for treatment. FBR against these implants can lead to complications such as malfunction, infection, soft tissue disfigurement, and pain. Acellular dermal matrix (ADM) has been used as a coating on implants to effectively attenuate FBR, despite an incomplete understanding of the underlying mechanisms. Given that ADM is expensive and not easily scalable, it is important to identify alternatives by uncovering its mechanisms of action.
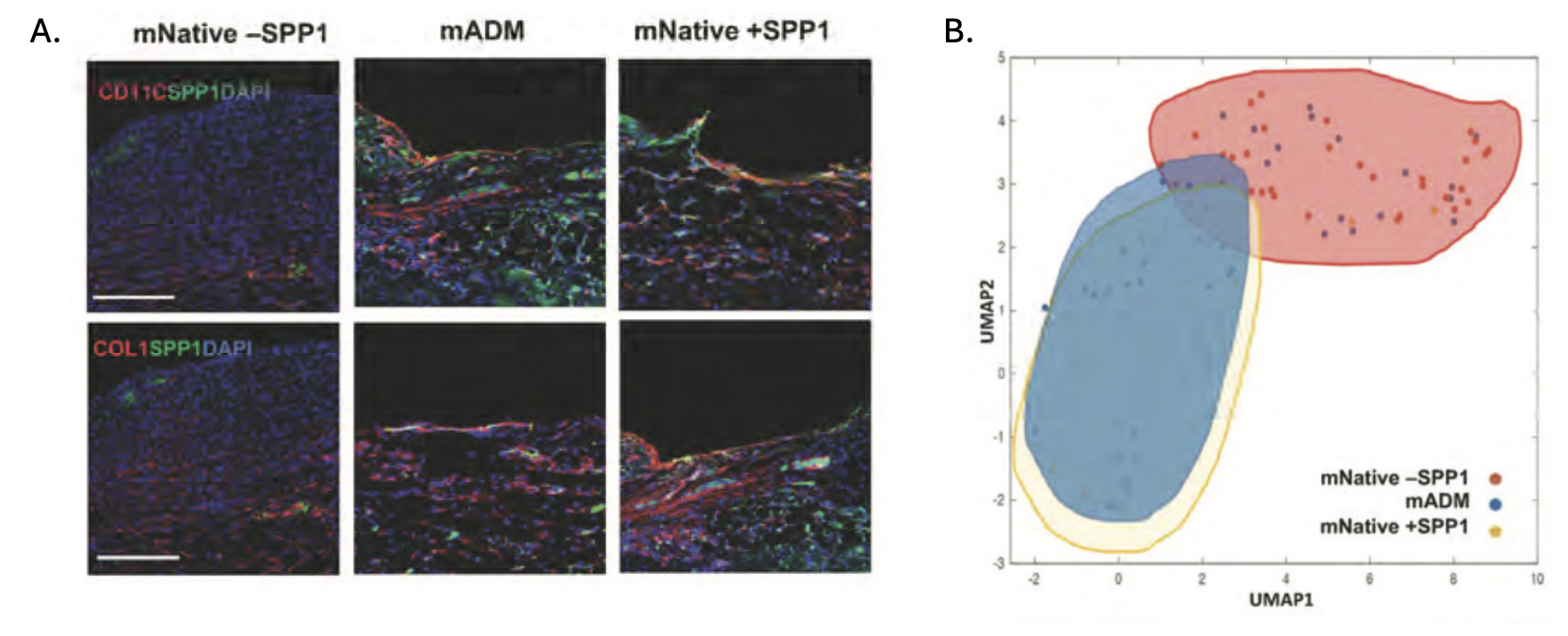
Stanford researchers have discovered that SPP1, upregulated by ADM, is necessary and sufficient for FBR attenuation. When a recombinant-SPP1-loaded hydrogel was placed adjacent to an implant, the fibrotic capsule was thinner compared to the conventional FBR. Additionally, the connective tissue architecture within the fibrotic encapsulation was comparable to that derived from ADM-coated implants. Directly managing FBR's main underlying driver SPP-1 could lead to significant improvements in biocompatibility of implantable devices.
Figure
Stage of Development
In vivo data
Applications
- Biomedical implants
- Biomedical devices
Advantages
- Cost effective
- Scalable
Publications
- Griffin, M., Tevlin, R., Parker, J., Liang, N., Guo, J., Valencia, C., Morgan, A., Kuhnert, M., Momeni, A., Wan, D., & Longaker, M. T. (2024). Acellular dermal matrix modulates monocyte transcriptome in mice and humans to attenuate foreign body response. Plastic & Reconstructive Surgery-Global Open, 12(S4), 42-43.
Related Links
Patents
- Published Application: WO2025049603
Similar Technologies
-
An Extrudable Biomaterial with Heat-Resistant Bioactivity and Tunable Degradation S23-458An Extrudable Biomaterial with Heat-Resistant Bioactivity and Tunable Degradation
-
Customizable, Porous Tissue Engineering Scaffold for 3D Cell Proliferation S12-089Customizable, Porous Tissue Engineering Scaffold for 3D Cell Proliferation
-
HyTEC: Hybrid Tissue Engineering Construct S20-447HyTEC: Hybrid Tissue Engineering Construct