Docket #: S12-346
Stable cycling of lithium sulfide cathodes through strong affinity with multifunctional binders
Rechargeable lithium sulfur batteries have attracted great interest in recent years because of their high theoretical specific energy, which is several times that of current lithium-ion batteries. Compared to sulfur, fully-lithiated Li2S represents a more attractive cathode material because it enables pairing with safer, lithium metal-free anodes.
Stanford researchers have designed and tested a new framework for a rational design of stable and high performance Li2S cathodes by using ab initio simulations to elucidate the interaction between Li2S and lithium polysulfides with various functional groups found in macromolecular binders. Using polyvinylpyrrolidone (PVP) as a binder in one embodiment, an initial specific capacity of about 760 mAh g-1 of Li2S (~1,090 mAh g-1 of S) was achieved at 0.2C, with unprecedented capacity retention of about 94% in the first 100 cycles. Even after prolonged cycling over 500 charge/discharge cycles, cells retained about 69% of their initial capacity, which corresponds to a small capacity decay of about 0.062% per cycle.
Figure 1
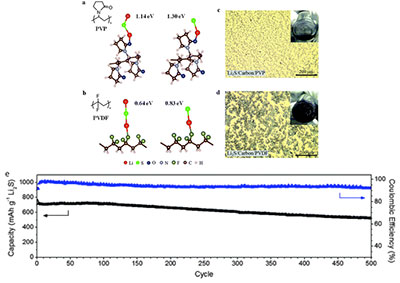
Figure 1 description -(a, b) Ab initio simulations showing the most stable configuration and calculated binding energies of Li2S and Li–S• species with (a) PVP and (b) PVDF binders. (c and d) Optical microscopy and digital camera images (inset) showing the electrode slurry of (c) Li2S /carbon black/PVP binder and (d) Li2S /carbon black/PVDF binder in N-methyl 2-pyrrolidinone (60:35:5 by weight in both cases) (e) Specific capacity and Coulombic efficiency of Li2S cathodes using PVP binder upon prolonged cycling over 500 cycles at 0.2 C.
Stage of Research
Applications
- Improved lithium sulfur battery performance for electronics, vehicles, energy storage
- Simple approach for modifying lithium sulfide cathode to achieve high energy density and long cycling
Advantages
- Low cost
- Simple fabrication
- Scalable production
- Improved specific capacity and cycle life
- Sulfur provides a 10x higher charge storage capacity
Publications
- Z. W. Seh, Q. Zhang, W. Li, G. Zheng, H. Yaoa and Y. Cui, "Stable cycling of lithium sulfide cathodes through strong affinity with a bifunctional binder," Chemical Science (2013).
Related Links
Patents
- Published Application: 20150010817
- Published Application: WO2015006279
- Issued: 10,033,040 (USA)
Similar Technologies
-
Nanoscale Interfacial Engineering with Interconnected Hollow Carbon Nanospheres for Stable Lithium Metal Anode S14-006Nanoscale Interfacial Engineering with Interconnected Hollow Carbon Nanospheres for Stable Lithium Metal Anode
-
Bamboo-like Carbon Nanofibers for Flexible Supercapacitors S15-096Bamboo-like Carbon Nanofibers for Flexible Supercapacitors
-
Nanocarbon/Inorganic Nanoparticle Hybrid Materials for Energy Storage and Fuel Cells S09-371Nanocarbon/Inorganic Nanoparticle Hybrid Materials for Energy Storage and Fuel Cells