Docket #: S20-117
Controlled Release of Bacteriophage to Treat Implant Infections
Stanford researchers have designed hydrogels that can be delivered to surgical sites in a patient's body for controlled and sustained release of bacteriophages to treat or prevent bacterial infections. This invention includes the hydrogel, a method for chemically binding the bacteriophages to the hydrogel, and a mechanism for sustained release of the bacteriophage over long times (from hours until up to a week). Additionally, the hydrogel containing the phages is not part of an implant but is an independent device. This device is being designed to be deployed locally (through injection or implantation) to treat bacterial infections therapeutically.
Figure
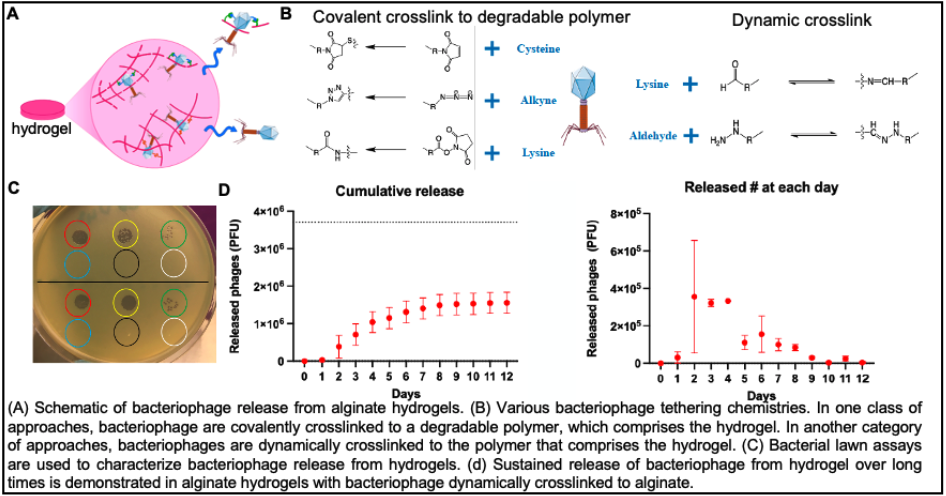
Image credit: All Innovators
Stage of Development
Applications
- Treatment and prevention of bacterial infections at surgical sites, wounds, or any other localized infection.
Advantages
- Bacteriophages have tremendous potential for treating bacterial infections, particularly those that are antibiotic resistant.
- Controlled release of bacteriophages
- Sustained release of bacteriophage over at least 7 days
- Hydrogel can also deliver phage cocktails and other agents
- Independent implant - gel is an independent implant in its own right
- New paradigm in implant-associated infection treatment
Related Links
Patents
- Published Application: WO2022204722
- Published Application: 20240173370
Similar Technologies
-
Use of Focal Adhesion Kinase Inhibitors To Improve the Quality and Appearance of Split Thickness Skin Grafts S20-006Use of Focal Adhesion Kinase Inhibitors To Improve the Quality and Appearance of Split Thickness Skin Grafts
-
Drug-imprinted hydrogel for controlled-release wound healing therapy with FAK inhibitors S14-447Drug-imprinted hydrogel for controlled-release wound healing therapy with FAK inhibitors
-
Deferoxamine prophylaxis for radiation-induced fibrosis S19-455Deferoxamine prophylaxis for radiation-induced fibrosis