Docket #: S20-006
Use of Focal Adhesion Kinase Inhibitors To Improve the Quality and Appearance of Split Thickness Skin Grafts
A team of researchers at Stanford have developed a hydrogel that delivers a scar-reducing focal adhesion kinase inhibitor (FAK-I) to skin grafts and donor sites.
Skin grafts are needed to treat burns and other injury, but leave patients with functional and aesthetic problems, including fragility, impaired joint function, and lack of hair, nerves, and pigmentation. To improve wound healing, the inventors have developed a pullaran and collagen hydrogel for local, sustained delivery of an FAK-I (see "Stanford Docket S14-447" for hydrogel design), which interferes with pro-fibrotic signaling in fibroblasts. This treatment improves skin regeneration in pig and mouse and is ready for rapid translation to the clinic.
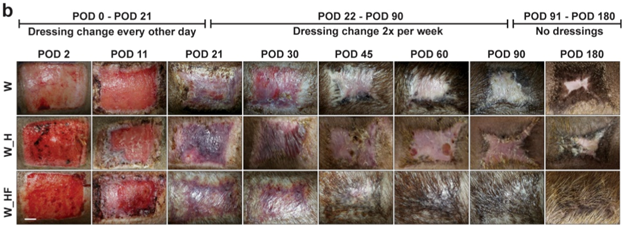
Photo description: Photographs of deep partial-thickness porcine excision wounds treated with placebo hydrogel (W_H) or FAK-I hydrogel (W_HF) show reduced scarring with FAK-I treatment. POD = post-operative day. Credit: Inventors
Stage of Development
Applications
- Hydrogel with controlled-release drug for split-thickness skin graft or donor site
- Scar reduction following burns, excisional surgery, or other skin injury
Advantages
- Acellular method to promote tissue regeneration
- Local, slow release avoids systemic side effects and frequent dressing changes
- Drug has known safety and efficacy in clinical trials (repurposed from cancer)
Publications
- Ma et al. Journal of Investigative Dermatology (2018) "Controlled delivery of a focal adhesion kinase inhibitor results in accelerated wound closure with decreased scar formation"
- Chen et al. Plastic Reconstructive surgery. Global Open (2020) "FAK inhibitor promotes tissue regeneration in porcine excision wounds"
Patents
- Published Application: WO2021194618
- Published Application: 20230121926
Similar Technologies
-
Controlled Release of Bacteriophage to Treat Implant Infections S20-117Controlled Release of Bacteriophage to Treat Implant Infections
-
Drug-imprinted hydrogel for controlled-release wound healing therapy with FAK inhibitors S14-447Drug-imprinted hydrogel for controlled-release wound healing therapy with FAK inhibitors
-
Deferoxamine prophylaxis for radiation-induced fibrosis S19-455Deferoxamine prophylaxis for radiation-induced fibrosis