Docket #: S23-514
Dual network, 3D printed pyrolytic carbon polymer structure fabrication method
The DeSimone Research Group at Stanford University developed a method for fabricating 3D pyrolytic carbon structures from polyacrylonitrile (PAN) generated by a scalable Vat Polymerization 3D-printing continuous liquid interface production (CLIP) process. PAN is popular for generating conductive high-strength carbon fibers commonly used in lightweight structural applications including battery systems for sustainable energy storage. However, because PAN is insoluble in its own AN monomer, it precipitates to form powder during VP 3D printing making it impossible to print. In response, the DeSimone Research Group developed this multi-step fabrication method for producing 3D carbon structures from PAN (See Figure below):
- A sacrificial crosslinked gel lattice scaffold is 3D CLIP printed.
- The gel scaffold is infused with acrylonitrile monomers and a thermal initiator.
- The acrylonitrile polymerizes inside the gel scaffold forming an interpenetrating polymer network.
- Finally, the PAN-infused gel lattice structure is thermally treated (e.g. an oxidative pre-treatment, isothermal holds, and/or pyrolysis) that converts the PAN to 3D pyrolytic carbon.
Figure
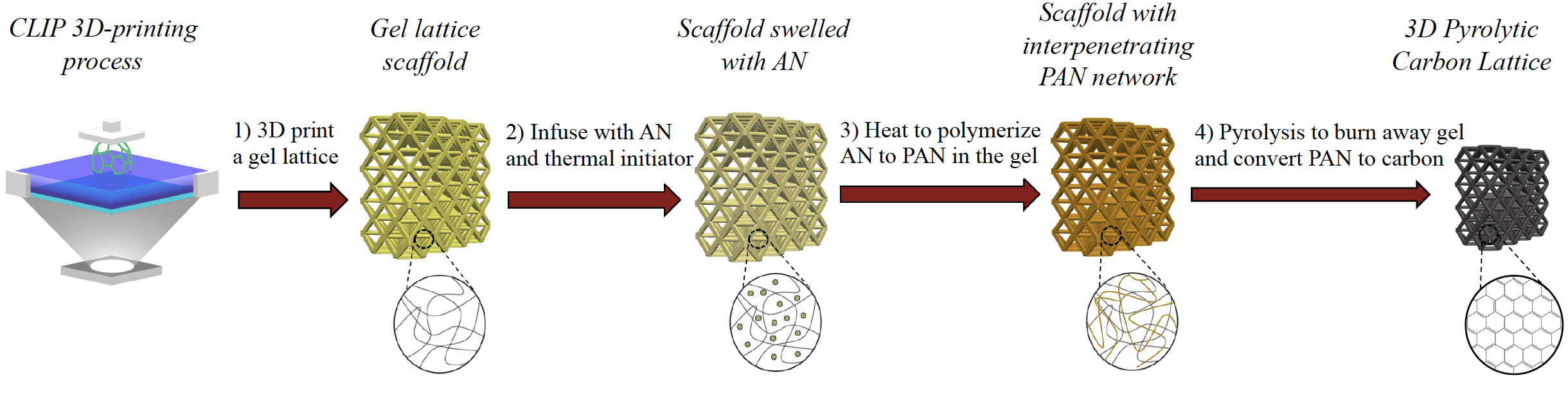
Figure Description: VP 3D-printing high-resolution PAN structures for pyrolysis
Image courtesy the DeSimone Research Group
The method incorporates PAN into 3D-printed high-resolution lattice structures via CLIP which following pyrolysis yields 3D lightweight electrode; This VP 3D-printing and gel infusion method can be used to pioneer the incorporation of other linear polymers, like polyethylene and polyimides, into high-resolution lattice structures.
Researchers at the Lu Group, UC Merced, have pioneered a method to create 3D pyrolytic carbon with an exceptionally high surface area, boosted by the inclusion of 1D carbon nanostructures. This method involves incorporating various catalysts either on the surface or within 3D printed polymers. The process enhances impingement through pressure, temperature, or evaporation-assisted techniques.
There are five roles these catalyst systems will fulfill:
- Promoting the formation of graphitic carbon precursor
- Coordination bonding to increase char yield
- Graphitization
- Carbon metal composite framework to prevent severe shrinkage during polymer pyrolysis, and enhance the mechanical integrity and electrical conductivity.
- The growth of 1D carbon nanostructures to enhance the electrical conductivity and surface area of the pyrolytic carbon.
Below is a process in steps:
- Catalyst and Biomass Precursor Infusion: The 3D printed template is immersed in a solution containing catalysts and biomass (such as sugar). During controlled solvent evaporation under elevated temperatures, the dehydrated biomass derivatives react with unreacted vinyl groups in the resin, forming carbon.
- Primary Structure Formation: The polymer template infused with catalyst undergoes pyrolysis under an inert atmosphere, solidifying the structure.
- Secondary Structure Growth: 1D carbon nanostructures are grown by introducing a gaseous carbon precursor, further increasing surface area and conductivity.
- Functionalization (Tertiary Structure): Electrografting tertiary species onto the secondary structure imbues the final product with application-specific functionalities.
Stage of Development – Proof of Concept
The Lu and DeSimone Research Groups are optimizing the process based on detailed electrochemical, mechanical, and surface topology characterization analyses to achieve optimal conductivity, char yield, and mechanical properties.
Applications
- Batteries for grid energy storage and EVs - 3D Printed, PAN-based pyrolytic carbon lattice structures for redox flow and EV batteries.
- CO2 recapture - pyrolytic carbon lattice electrocatalysis for high-throughput reactions such as the reverse water gas shift (RWGS) reaction and other reactions requiring high volumetric-density electrochemically active surface area and to facilitate mass transport.
- Bio and environmental sensing.
- Chemical production via electrification.
- Support for electrocatalysis to enable hydrogen economy.
Advantages
- The secondary and tertiary structures enable the extremely high volumetric chemical reaction hitherto unattainable.
- First in class fabrication of acrylonitrile swelled and polymerized in a 3D-printed gel structure followed by pyrolysis to create revolutionary 3D lattice carbon structures.
- Excellent electrical conductivity, strong chemical stability, and excellent mechanical properties.
- Quick, high resolution, scalable Vat Polymerization 3D-printing via CLIP.
- One step pyrolysis process combining graphitization and secondary carbon nanostructure growth.
- The multifunctionality of the catalysts is the key for highly electrically conductive, mechanically robust, 3D structures that offer easily accessible, extremely large surface areas.
- Green fabrication through minimal chemical usage when biomass is used and hierarchical carbon nanostructure is formed in situ.
Related Links
Patents
- Published Application: WO2025151617
Similar Technologies
-
Denoising WaveY-Net: An ultra-fast, auxiliary neural network enhanced surrogate field solver S22-445Denoising WaveY-Net: An ultra-fast, auxiliary neural network enhanced surrogate field solver
-
Thermoresponsive Material to Prevent Battery Fire S15-458Thermoresponsive Material to Prevent Battery Fire
-
Simple synthesis of uniform carbon flower superstructures for electrocatalysts and other energy and environment applications S18-114Simple synthesis of uniform carbon flower superstructures for electrocatalysts and other energy and environment applications