Docket #: S18-551
Flow Batteries with High Energy Density Redox-active eutectic liquid
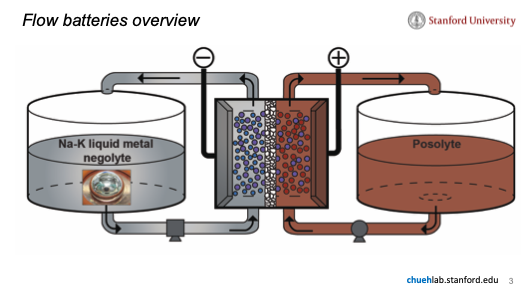
Flow batteries have an attractive battery architecture due to their scalability, long cycle-life, and power-to-energy tunability. However, they suffer from very low energy density (1/10th that of Li-ion batteries) due to the weight and volume of the water in their anolytes and catholytes and are only practical for grid-storage.
Engineers at the Chueh Lab have proposed a solution by creating a high-energy density catholyte or anolyte that can be incorporated into next-generation flow batteries for cost-effective energy storage. By leveraging the mixing behavior of different types of redox active molecules, a very high energy-density catholyte or anolyte can be made without the expense and added weight of incorporating solvents or long sidechains.
Higher energy density flow batteries can expand applications beyond grid storage to powering ships, locomotives, and vehicles and storing wind and solar energy.
Figure
Stage of Research
- Demonstrated the reduction in melting points using benzoquinone derivatives, with preliminary data on redox behavior
- Continued work to demonstrate cycling of these mixtures
Applications
- Flow batteries for grid storage, solar and wind power storage, and electrical vehicles and other locomotive power.
- Fuel cells
Advantages
- Enables lower cost, high density flow batteries
- 5-10X higher energy density as compared to conventional catholytes and anolytes
- All components are redox-active without the use of a solvent, allowing higher density.
- Can create room temperature liquid catholytes or anolytes via eutectic behavior, i.e. the reduction in melting points via mixing
- New applications - A flow battery with an energy density comparable to Li-ion batteries can unlock applications such as electric vehicles, accelerating the electrification of transportation and allowing greater adoption of flow batteries to store wind and solar energy
Stanford News, July 2018
Related Links
Patents
- Published Application: 20200243912
- Issued: 11,450,889 (USA)
Similar Technologies
-
Low-cost, durable anode to split seawater for efficient renewable energy storage S18-035Low-cost, durable anode to split seawater for efficient renewable energy storage
-
Digital Twin Platform for 24/7 Carbon-Free Electrified Fleet Operations S23-398Digital Twin Platform for 24/7 Carbon-Free Electrified Fleet Operations
-
Powernet: Behind-the-Meter Resource Management System for Farms S21-074Powernet: Behind-the-Meter Resource Management System for Farms