Docket #: S17-342
High efficiency electrocatalysis with lung-inspired architecture
Stanford researchers have developed a low-cost, highly efficient system for performing a wide range of electrocatalytic reactions. This technology is designed to mimic the topology of the human lung in order to quickly deliver gas to and from the catalytic surface, thereby enhancing performance.
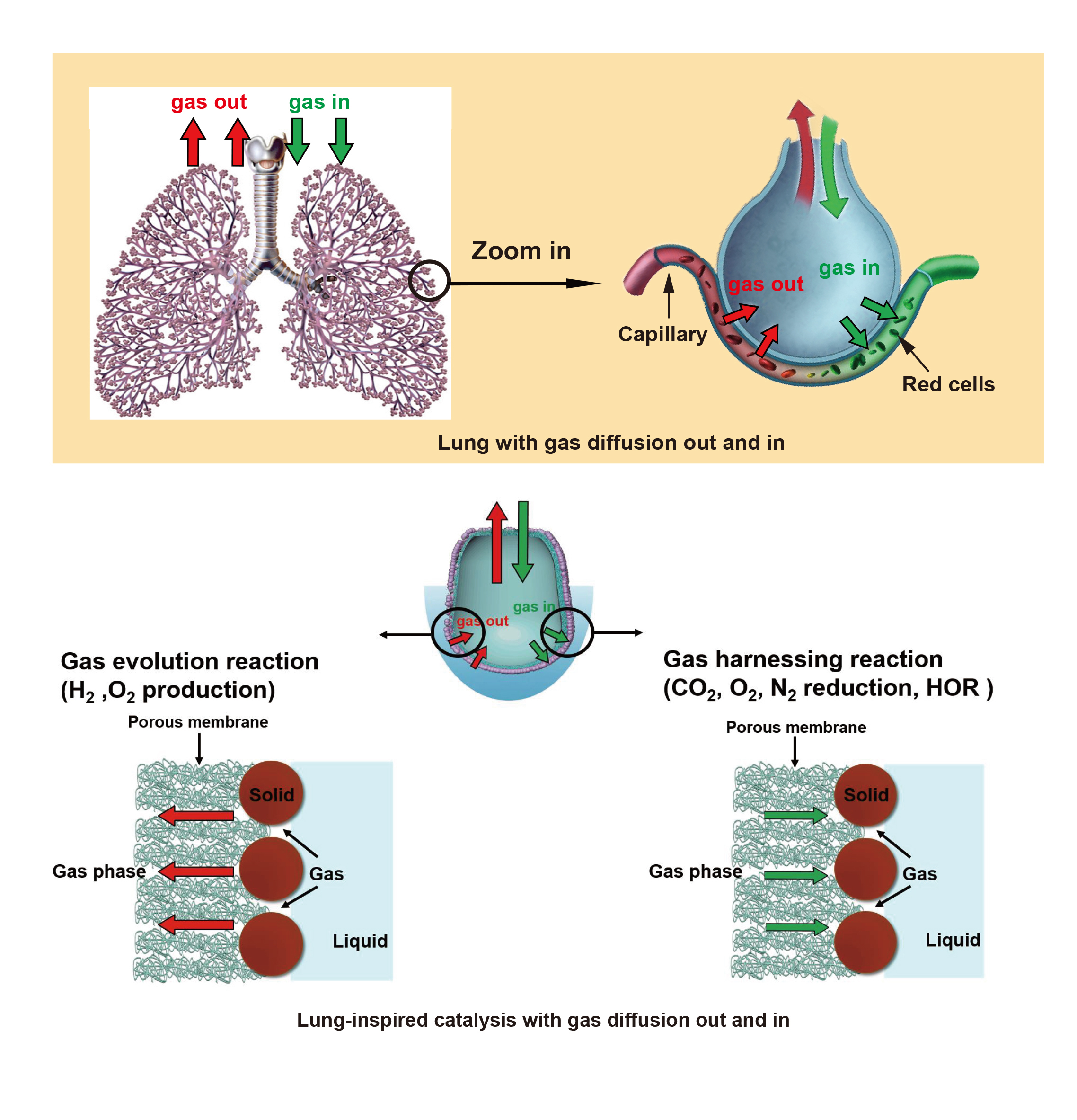
The general structure is formed by a flexible, micron-scale nanoporous membrane that is permeable to gas and not liquid. This membrane is coated with an ultra-thin layer of catalyst and creates a gas-liquid-solid (G-L-S) three-phase interface for highly efficient gas exchange, facilitating a wide range of reactions depending on the catalyst chosen. This compact structure could improve performance of electrocatalytic devices in a broad range of clean energy applications such as fuel cells, metal-air batteries, electrochemical water splitting and CO2 reduction.
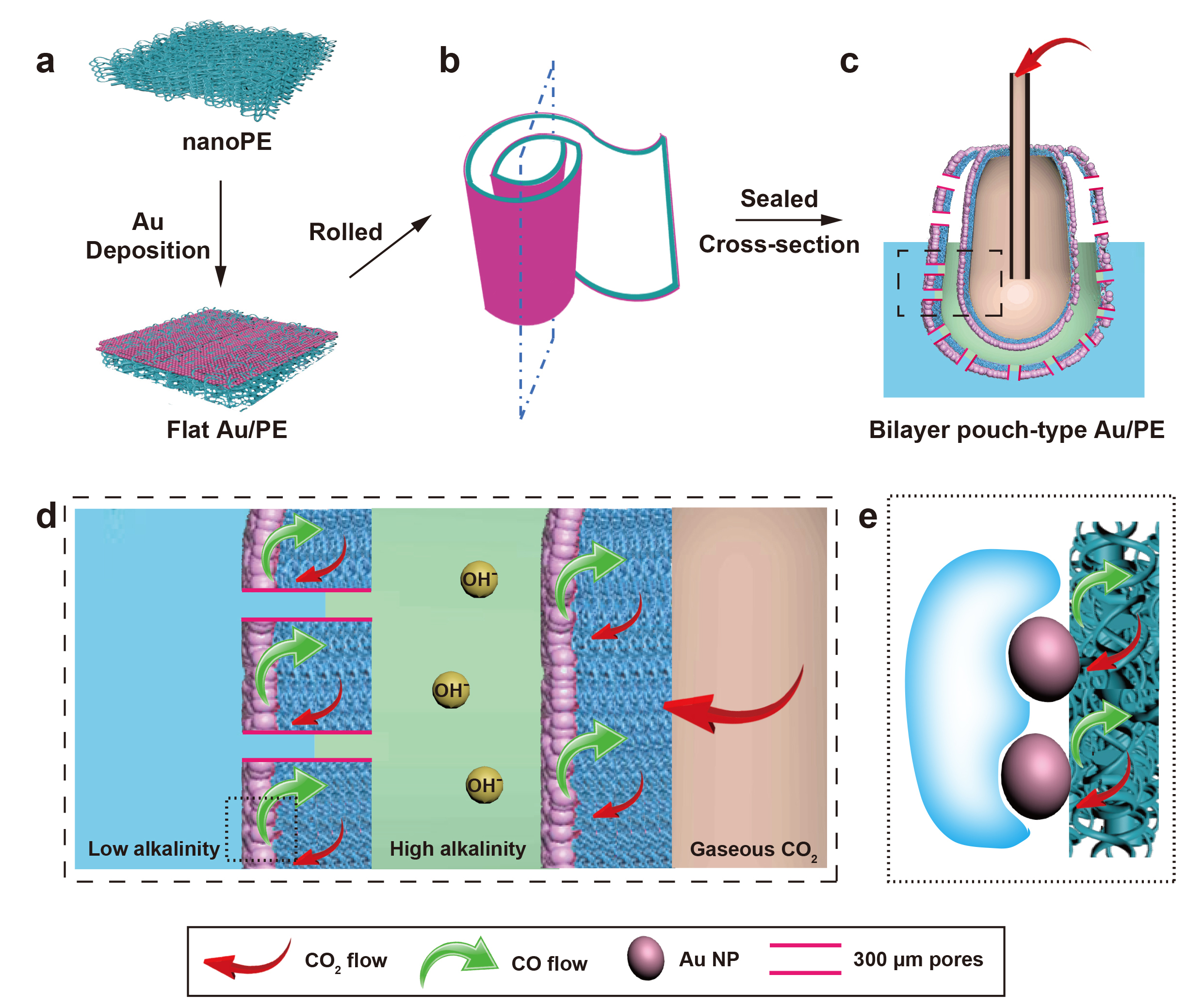
Schematics of the lung-inspired system for efficient electrocatalytic CO2 reduction. A layer of Au catalyst is sputtered on a nanoporous polyethylene membrane (nanoPE), the Au-coated PE membrane is rolled up and sealed into a bilayered structure mimicking a human lung. CO2 gas is delivered to the central compartment of the Au/PE membrane. Electrochemical reduction takes place on the electrolyte-catalyst-gas interface while the penetration and reduction of water molecules are inhibited. By varying the catalyst, this basic structure and function can also enhance performance of oxygen reduction, oxygen evolution or other reactions.
Stage of Research
The inventors have demonstrated performance in a variety of reactions and are investigating more electrocatalytic systems to establish the benefits of this architecture. Systems tested include:
Carbon Dioxide Reduction (CDR) with Au/PE catalyst vs. reversible hydrogen electrode (RHE) – showed CO production selectivity of ~92% faradic efficiency at -0.6V, current density of CO production of ~25.5mA cm-2 at-0.6V, and current density of CO production ~1 mA cm-2 at 0.8V
Oxygen Reduction Reaction (ORR) with Ag/Pt bilayer catalyst: showed current density of 250mA cm-2 at 0.6V (~25X higher current densities than open electrode structure)
Oxygen Evolution Reaction (OER) with Au/NiFeOx catalyst: showed record low overpotential of 190 mV at 10mA cm-2 (30-90 mV lower than flat electrode)
Applications
- Clean energy electrocatalytic devices - gas exchange architecture to optimize catalytic performance for reactions with three phase contact (solid catalyst, liquid, and gas) for:
- reactions such as CO2 reduction reaction (CDR), oxygen reduction reaction (ORR), oxygen evolution reaction (OER), hydrogen evolution reaction (HER), hydrogen oxidation reaction (HOR), and nitrogen reduction reaction (NRR)
- end user applications such as fuel cells, metal air batteries, carbon dioxide reduction, hydrolysis, gas separation
Advantages
- Highly efficient - design allows gas reactants and products to be delivered continuously through to the three-phase contact interface, thus enabling full utilization of almost all the catalyst atoms, with results such as:
- increased catalytic activity from high flux CO2 delivery in carbon reduction reaction
- record low overpotential for oxygen evolution reaction as newly formed O2 molecules quickly diffuse into the gas phase, waiving the energy cost of bubble nucleation
- 25X higher current densities than open electrode structure for oxygen reduction reaction with significantly enhanced catalytic performance through increased available O2 flux to the electrocatalytic solid-liquid-vapor interface
- Resistance to flooding – compared with conventional carbon-based gas diffusion electrode, the nanoPE showed excellent resistance to electrolyte flooding which will hinder the mass transport and result in poor durability and degradation.
- Low cost:
- ultrathin catalyst layer (50-100nm) with extremely high mass activity reduces cost of materials
- nanoPE can be mass-produced via roll-to-roll fabrication
- Versatile, tunable design - general membrane-enclosed structure can be tuned by size, catalyst and material to optimize for different conditions and electrochemical reactions
- Compact electrodes - enables applications with space constraints (e.g., auto, home, aircraft)
Publications
- J. Li, G. Chen, Y. Zhu, Z. Liang, A. Pei, C.-L. Wu, H. Wang, H. R. Lee, K. Liu, S. Chu, and Y. Cui. Efficient electrocatalytic CO2 reduction on a three-phase interface. Nature Catalysis. 23 July 2018 DOI:10.1038/s41929-018-0108-3
- J. Li, Y. Zhu, W. Chen, Z. Lu, J. Xu, A. Pei, Y. Peng, X. Zheng, Z. Zhang, S. Chu, and Y. Cui, Breathing-Mimicking Electrocatalysis for Oxygen Evolution and Reduction. Joule (2018) DOI: 10.1016/j.joule.2018.11.015
Related Links
Patents
- Published Application: 20210198795
Similar Technologies
-
Low-cost, durable anode to split seawater for efficient renewable energy storage S18-035Low-cost, durable anode to split seawater for efficient renewable energy storage
-
Oxygen tolerant hydrogenases by mutating electron supply pathway S15-300Oxygen tolerant hydrogenases by mutating electron supply pathway
-
Materials for low cost, scalable, thermochemical hydrogen production S16-325Materials for low cost, scalable, thermochemical hydrogen production