Docket #: S24-200
High-Efficiency Microneedle Array System for Rapid Dermal Interstitial Fluid Collection
Stanford scientists have developed a novel microneedle array system that rapidly collects dermal interstitial fluid for diagnostic applications. This minimally invasive technology consistently extracts 10 ?L of ISF in just 5 minutes—5-10 times faster than current methods—providing efficient access to biomarkers comparable to those found in blood plasma.
Dermal interstitial fluid (ISF) represents an untapped resource for biomedical research and diagnostics, containing valuable biomarkers comparable to those found in blood while offering potential advantages over traditional blood draws. Despite its promise, ISF research has been severely constrained by collection methods that are either too invasive, requiring anesthesia and lengthy recovery times, or too inefficient, yielding insufficient volumes for comprehensive analysis. These limitations have restricted ISF studies to small cohorts, with the largest investigations including only dozens of participants. The field urgently needs practical collection technologies that can enable larger-scale research and unlock new diagnostic applications for skin and lymphatic diseases.
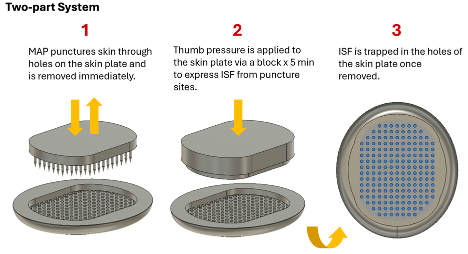
The disclosed microneedle array system collects ISF by creating optimal pressure gradients when applied to skin tissue. Drawing on groundwater engineering principles, the device's carefully designed geometry maximizes fluid flow to collection sites, achieving 5-10 times faster collection rates than existing approaches. The system pairs a microneedle array that creates precise skin punctures with a specialized collection plate that efficiently captures expressed fluid through capillary action. Importantly, this design eliminates the need for external implements like vacuum pumps or capillary tubes, making it practical for widespread clinical use. This innovation opens the door to both expanded research applications and new diagnostic capabilities by providing reliable, efficient access to this valuable biological fluid.
Figure
Stage of Development:
Prototype
Continued research – A pilot human trial using the device is currently ongoing.
Applications
- Development of less invasive diagnostics as alternatives to blood draws
- Fundamental research on ISF biomarkers and exosome content
- Testing for skin and lymphatic diseases using locally derived fluid
- Enabling larger-scale human trials for ISF research
- Integration with wearable sensors and lateral flow assays
Advantages
- 5-10x faster collection (10 ?L in 5 minutes) than existing methods
- Self-contained design with no external implements required
- Minimally invasive with rapid skin recovery
- Optimized geometry for maximum fluid collection efficiency
- Customizable collection parameters for different applications
Publications
- Andy H. Hung, Netra U. Rajesh, Abel Bermudez, Stephanie M. Boczek, Fernando J. Garcia-Marques, Yee Lin Tan, Jihyun (Luna) Hwang, Prima Dewi Sinawang, Dan Ilyin, Gunilla B. Jacobson, Utkan Demirci, Steven P. Poplack, Sharon J. Pitteri, Joseph M. DeSimone. (2025) A Microneedle Device for Rapid Dermal Interstitial Fluid Sampling. bioRxiv 2025.03.13.641882.
- Ribet, F., Bendes, A., Fredolini, C., Dobielewski, M., Böttcher, M., Beck, O., ... & Roxhed, N. (2023). Microneedle patch for painless intradermal collection of interstitial fluid enabling multianalyte measurement of small molecules, SARS?CoV2 antibodies, and protein profiling. Advanced Healthcare, 12(13), 2202564.
- Samant, P. P., Niedzwiecki, M. M., Raviele, N., Tran, V., Mena-Lapaix, J., Walker, D. I., ... & Prausnitz, M. R. (2020). Sampling interstitial fluid from human skin using a microneedle patch. Science translational medicine, 12(571), eaaw0285.
- Miller, P. R., Taylor, R. M., Tran, B. Q., Boyd, G., Glaros, T., Chavez, V. H., ... & Polsky, R. (2018). Extraction and biomolecular analysis of dermal interstitial fluid collected with hollow microneedles. Communications biology, 1(1), 173.
Related Links
Similar Technologies
-
Robust 3D printed pyrolytic carbon micro-array patch for transdermal applications S23-282Robust 3D printed pyrolytic carbon micro-array patch for transdermal applications
-
A suprachoroidal spacer implant to treat glaucoma S23-174A suprachoroidal spacer implant to treat glaucoma
-
Development of SDF1alpha containing nanoparticles for the treatment of cardiovascular, Neurovascular, and skin impairments S23-446Development of SDF1alpha containing nanoparticles for the treatment of cardiovascular, Neurovascular, and skin impairments