Docket #: S18-217
Non-invasive Blood Test for Diagnosing Crohn's Disease and Ulcerative Colitis
Diagnosis and sub-typing of inflammatory bowel disease (IBD) subsets, such as Crohn's disease (CD) and ulcerative colitis (UC), often require the use of repeated, invasive, and expensive endoscopy procedures, which are not without risk. Furthermore, 10–20% of patients with indeterminate colitis are misdiagnosed because it is difficult to differentiate CD from UC when disease is confined to the colon, but each condition benefits from distinct therapeutic approaches.
Researchers at Stanford have developed a non-invasive method, based on the identification of novel immune signatures in the blood, for diagnosing CD or UC in patients with IBD. Misdiagnosis and delayed diagnosis due to time required for endoscopy and histopathology analysis of biopsies remain serious problems that delay optimal treatment, representing a critical unmet need. Earlier treatment leads to better outcomes, and treatment for patients with active disease is frequently delayed by the time required for differentiation of CD versus UC by these invasive and costly procedures. In contrast, the new method may be implemented easily on existing equipment using a single fresh or frozen blood specimen, potentially enabling earlier and more definitive diagnosis, as well as with minor adaptations better monitoring and precision treatment of IBD patients. The blood-based diagnostic signatures are non-invasive, low risk, cost-effective, and highly accurate for differentiating Crohn's disease from ulcerative colitis, including the challenging Crohn's disease confined to the colon from ulcerative colitis.
Figure
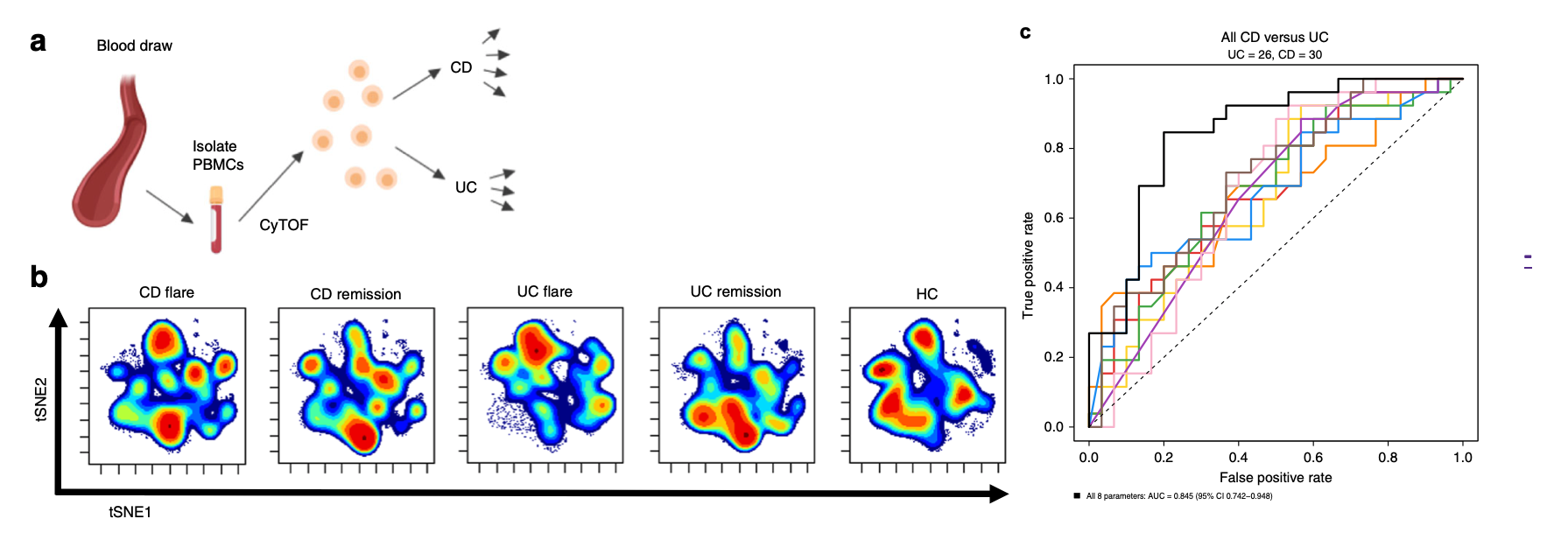
Stage of Development: Proof of Concept
The researchers have identified several blood-based signatures that differentiate CD from UC, and most importantly perform similarly to the standard-of-care endoscopy/colonoscopy for patients who have active disease – the most significant clinical unmet need. They have developed the diagnostic markers on a training patient cohort using mass cytometry (CyTOF) and validated the discriminatory performance on an independent validation patient cohort dataset using conventional clinical flow cytometry. They continue to study the markers on additional patients and optimize the diagnostic model to differentiate other clinically-relevant subtypes of IBD and predict therapeutic response to improve precision medicine in IBD and save patients and the healthcare system time, cost and adverse effects.
Applications
- Blood-based diagnosis of inflammatory bowel disease
- Crohn's disease diagnosis
- Ulcerative colitis diagnosis
- Monitoring disease activity
- Prediction of therapeutic response
- Colon disease diagnosis
Advantages
- Method is less invasive, safer and cheaper than standard-of-care endoscopy/colonoscopy.
- Potentially more sensitive and specific than standard-of care endoscopy/colonoscopy, serology, and stool markers.
- Can be performed on flow cytometers commonly found in clinical diagnostic labs.
- Could be introduced clinically to reduce delay, risk, discomfort and cost to patients, and improve outcomes by arriving at a definitive diagnosis faster.
- Could be easily adapted as a companion diagnostic for existing or emerging therapies.
Publications
- Samuel J. S. Rubin, Lawrence Bai, Yeneneh Haileselassie, Gotzone Garay, Chohee Yun, Laren Becker, Sarah E. Streett, Sidhartha R. Sinha & Aida Habtezion (2019). Mass cytometry reveals systemic and local immune signatures that distinguish inflammatory bowel diseases. Nature Communications 10, Article number: 2686 (published 19 June 2019).
- Gottfried-Blackmore A, Rubin SJS, Bai L, Aluko S, Yang Y, Park W, Habtezion A. (2020).Effects of processing conditions on stability of immune analytes in human blood." Sci Rep. 2020 Oct 15;10(1):17328.
- Fenton TM, Jørgensen PB, Niss K, Rubin SJS, Mörbe UM, Riis LB, Da Silva C, Plumb A, Vandamme J, Jakobsen HL, Brunak S, Habtezion A, Nielsen OH, Johansson-Lindbom B, Agace WW (2020). Immune Profiling of Human Gut-Associated Lymphoid Tissue Identifies a Role for Isolated Lymphoid Follicles in Priming of Region-Specific Immunity. Immunity. 2020 Mar 17;52(3):557-570.e6. doi: 10.1016/j.immuni.2020.02.001. Epub 2020 Mar 10. PMID: 32160523; PMCID: PMC7155934.
- Gubatan J, Rubin SJS, Bai L, Haileselassie Y, Levitte S, Balabanis T, Patel A, Sharma A, Sinha SR, Habtezion A. (2021)."Vitamin D Is Associated with ?4?7+ Immunophenotypes and Predicts Vedolizumab Therapy Failure in Patients with Inflammatory Bowel Disease." J Crohns Colitis. 2021 Dec 18;15(12):1980-1990.
- Bai L, Dermadi D, Kalesinskas L, Dvorak M, Chang SE, Ganesan A, Rubin SJS, Kuo A, Cheung P, Donato M, Utz PJ, Habtezion A, Khatri P (2023). Mass-Cytometry-Based Quantification of Global Histone Post-Translational Modifications at Single-Cell Resolution Across Peripheral Immune Cells in IBD. J Crohns Colitis. 2023 May 3;17(5):804-815. doi: 10.1093/ecco-jcc/jjac194. PMID: 36571819; PMCID: PMC10155749.
- Holman DR, Rubin SJS, Ferenc M, Holman EA, Koron AN, Daniel R, Boland BS, Nolan GP, Chang JT, Rogalla S. (2024). Automated spatial omics landscape analysis approach reveals novel tissue architectures in ulcerative colitis. Sci Rep. 2024 Aug 15;14(1):18934. doi: 10.1038/s41598-024-68397-5. PMID: 39147769; PMCID: PMC11327370.
- Kotagiri P, Rae WM, Bergamaschi L, Pombal D, Lee JY, Noor NM, Sojwal RS, Rubin SJS, Unger LW, Tolmeijer SH, Manferrari G, Bashford-Rogers RJM, Bingham DB, Stift A, Rogalla S, Gubatan J, Lee JC, Smith KGC, McKinney EF, Boyd SD, Lyons PA. (2025).Disease-specific B cell clones are shared between patients with Crohn's disease. Nat Commun. 2025 Apr 17;16(1):3689. doi: 10.1038/s41467-025-58977-y. PMID: 40246842; PMCID: PMC12006383.
Related Links
Patents
- Published Application: WO2020142276
- Published Application: 20210396753
- Issued: 12,461,101 (USA)
Similar Technologies
-
Engineering the Gut Microbiome for Imaging and Therapeutic Uses S14-452Engineering the Gut Microbiome for Imaging and Therapeutic Uses
-
Fluorescent saccharide sensors for early detection of gastrointestinal diseases S17-396Fluorescent saccharide sensors for early detection of gastrointestinal diseases
-
A Non-Invasive Diagnostic Platform for Precise, Biomarker-Based Detection of Active Bacterial Infections S24-420A Non-Invasive Diagnostic Platform for Precise, Biomarker-Based Detection of Active Bacterial Infections