Docket #: S16-365
High-specificity proximity ligation assay to enable detection with low-affinity agents
Researchers at Stanford Genome Technology Center have patented a highly sensitive and specific straightforward circular Proximity Ligation Assay (c-PLA) method to reduce background and improve quantitative detection of protein biomarkers through conversion into unique DNA sequences. This invention greatly improves signal-to-background ratio by using two oligonucleotide molecules that ligate to form a new circular DNA molecule. After ligation the circular DNA molecules are isolated and used for either direct qPCR readout or library preparation and subsequent NGS readout. The additional proof-reading step enabled through DNA complementarity increases the specificity of the assay by suppressing random background ligation events and makes the assay compatible with low affinity capture reagents. Genomics workflow of c-PLA allows for quantitative detection of biomarkers via qPCR for singleplex readout or NGS for multiplex readout consuming only 2 µL sample volume. This technology expands the utility of PLA for a variety of research and diagnostic applications including integration with multi-omics/liquid biopsy field, proximity-based detection and transplant diagnostics/HLA antibody detection.
Figure
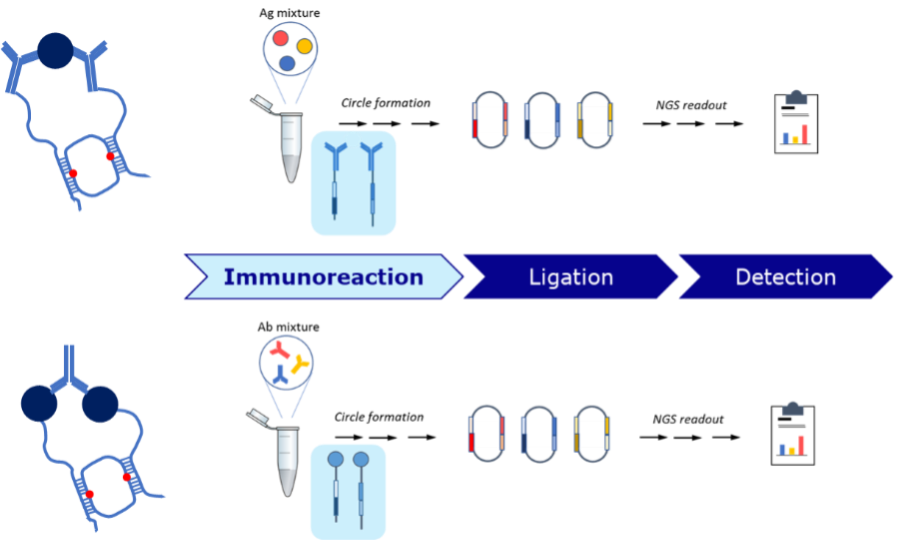
Stage of Development: Proof of Concept
Inventors have accomplished quantitative detection of both antigens and antibodies in plasma via c-PLA and showed the technology has superior performance metrics over ELISA, PLA and PEA (Olink) in terms of specificity (low background and high precision CV 15%), sensitivity (femtomolar LOD), covering wide dynamic range (> 3 logs), ease of use, and compatibility with low affinity reagents2.
They have optimized assay for a panel of more than a dozen plasma proteins, including cancer markers, achieving detection within relevant physiological ranges and enabling simultaneous quantification from femtomolar to nanomolar concentrations in a single 2 µL sample.
Applications
- Protein detection - PLA-based assay to detect low quantities of analytes (e.g., proteins, peptides, drugs, metabolites) in blood with end user applications in research and diagnostics including integration with multi-omics/liquid biopsy field, proximity-based detection and transplant diagnostics/HLA antibody detection.
Advantages
- High specificity with low background – Additional proof-reading steps through DNA complementarity improves specificity (suppresses background), and allows for an increase in capture-probe concentration thereby improving signal-to-noise ratio
- Enables detection using low affinity capture agents (e.g., antibodies, or aptamers with low affinity (high equilibrium dissociation constant, KD)
- Eliminates cross reactivity due to increased specificity
- Decreases assay variability (high precision: CVs 15%)
- High sensitivity – Amplification of DNA results in higher signal generation and improved sensitivity
- Improves assay limit-of-detection (down to femtomolar concentration) due to higher signal with suppressed background noise
- Small sample volume – consumes low volume of reagents and samples
- Lowers reagent cost and precious sample analysis
- NGS readout – enables digital quantitation and wide dynamic range
- Facilitates detection within relevant physiological ranges (> 3 logs dynamic range)
- Scalability of DNA assays – enables massive parallelization with NGS
- Allows for multiplexing of both target proteins and samples
- Genomics workflow – provides combined readout with DNA/RNA
- Facilitates integration with multi-omics applications for quantitative detection of proteins, DNA and RNA in a single detection platform
Publications
- Jalili, R., Horecka, J., Swartz, J. R., Davis, R. W., & Persson, H. H. (2018). Streamlined circular proximity ligation assay provides high stringency and compatibility with low-affinity antibodies. Proceedings of the National Academy of Sciences, 115(5), E925-E933.
Patent Status
Patent issued:
- Europe: 3589750 (May 4, 2022)
- Japan: 7248368 (March 29, 2023)
- United States: 11,530,438 (December 20, 2022)
Related Links
Patents
- Published Application: WO2018160397
- Published Application: 20190360025
- Published Application: 20230295691
- Issued: 11,530,438 (USA)
Similar Technologies
-
Method for Improved Pathogen Detection S18-367Method for Improved Pathogen Detection
-
Composition and Methods for Transglutaminase-2 Mediated Endocytosis S22-345Composition and Methods for Transglutaminase-2 Mediated Endocytosis
-
Fluorescent saccharide sensors for early detection of gastrointestinal diseases S17-396Fluorescent saccharide sensors for early detection of gastrointestinal diseases