Docket #: S24-130
Plasmonic gas diffusion reactor for CO2 conversion to high-value chemicals
Industry, government, and private investment in CO2 capture is growing to address climate change. Without carbon utilization, however, high costs impede large scale capture efforts. Alexander Al Zubeidi, a Stanford post doc in the D-Lab, has developed an inexpensive, scalable gas flow cell based system to convert atmospheric CO2 to other hydrocarbon based chemicals (like ethylene) using light and excess renewable electricity.
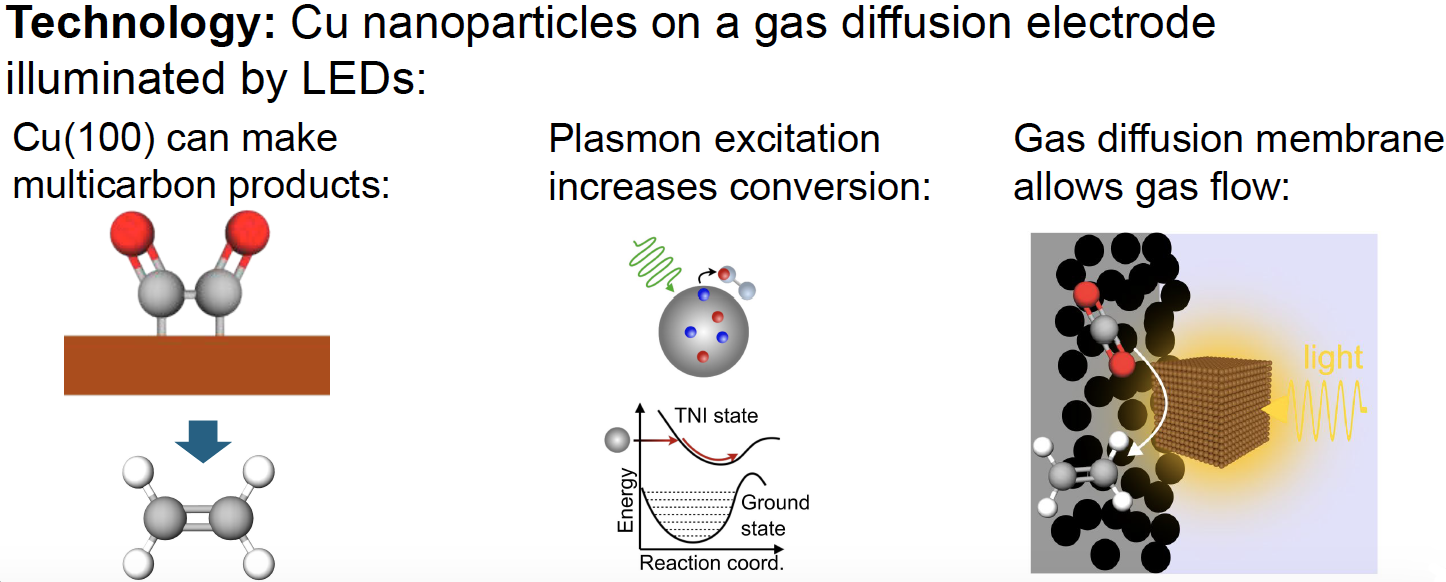
Figure 1 – Prototype Cell Process
(Image courtesy the D-Lab)
In the prototype system, gas enters the reactor cell via a gas flow channel, flows over the gas diffusion electrode covered in copper nanoparticles and electrolyte solution at ambient temperature. Visible light (450-800 nm) enters through the cell window, exciting copper nanoparticle electrons that reduce CO2 to ethylene. These electrolyzers can produce hydrocarbon based chemicals and syngas, a mixture of H2 and O2. Unlike competing electrolyzers that are built to operate on large scales, with long payback periods that typically require high capacity factors, the D-Lab system (Figure 2) can operate when renewable energy is in excess, generating net-zero emissions and converting point-source CO2 emissions to high-value products. This inexpensive, scalable plasmonic gas flow reactor system provides cost effective carbon capture CO2 gas separation and storage while producing valuable feedstocks for the chemical industry or zero-carbon fuels.
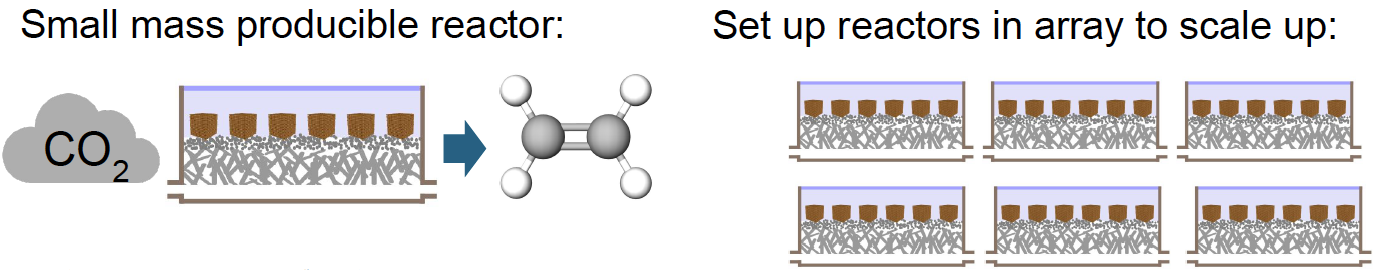
Figure 2 – Scaled Up Reactor Array
(Image courtesy the D-Lab)
Stage of Development – Proof of Concept Prototype
Applications
- Industrial chemical production, especially ethylene
- Green fuel / hydrocarbon based fuel production
- Syngas production
Advantages
- Low cost ethylene production with high cap-ex return: A 20 x 20 cm2 reactor operating at 80% selectivity for ethylene at 0.5 A/ cm2 operated 6 h a day can produce enough ethylene in 1 month to pay for itself.
- Compact, scalable and mass producible: Easy to ship and scale up using eletrolyzers in parallel to keep production and supply chain uncomplicated.
- No purification of reactants: The gas diffusion electrode operates with captured CO2 and gas mixtures containing CO2, which reduces upfront costs and energy consumption.
- Operates at ambient temperature: The electrolyzer can be started and shut down rapidly, allowing it to only operate when electricity costs are low.
- Does not require CH3
Publications
- Stanford Report: Sustainability Accelerator - The first cohort of the accelerator's new postdoc fellowship program for innovators will focus on the challenges of removing billions of tons of greenhouse gases from Earth's atmosphere by 2050
Related Links
Patents
- Published Application: WO2026011140
Similar Technologies
-
Materials for low cost, scalable, thermochemical hydrogen production S16-325Materials for low cost, scalable, thermochemical hydrogen production
-
Biomimetic Sorbents for CO2 Capture S12-500Biomimetic Sorbents for CO2 Capture
-
Delivery and Enzymatic Conversion of Hydrogen Gas to Reducing Equivalents for Carbon-Negative Biosynthesis S21-276Delivery and Enzymatic Conversion of Hydrogen Gas to Reducing Equivalents for Carbon-Negative Biosynthesis