Docket #: S16-325
Materials for low cost, scalable, thermochemical hydrogen production
Engineers in Prof. Arunava Majumdar's laboratory have formulated high-entropy phase-change materials that can split water to produce hydrogen at moderate temperatures using a scalable, carbon-free process. The hydrogen is produced through a two-step solar-powered thermochemical redox reaction. Then it can be harnessed to reduce carbon dioxide and produce chemicals such as plastics, syngas or transportation fuel.
The reaction is compatible with the existing infrastructure of the chemical industry reaction because it proceeds at moderate temperatures (~1200oC or lower) with extremely fast oxygen release kinetics. In addition, the materials used for the reaction are in powder form, allowing the patented process to be scaled volumetrically in well-known chemical reactor designs (e.g. fluidized bed). This water-splitting technology is designed to potentially produce carbon-free hydrogen at prices competitive with steam methane reforming (SMR). Furthermore, it may turn CO2 from an environmental liability to an asset by converting it CO feedstock for chemical and renewable fuel production.
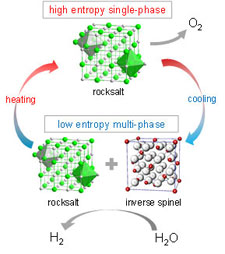
Schematic diagram of two-step thermochemical water splitting reactions using the proposed high-entropy mixed oxides.
Stage of Development
The inventors have formulated the entropy stabilized material, verified that it can produce hydrogen at 1200oC or lower (lower temperature than current state of the art), and performed repeatability studies to ensure that it can be cycled continuously to produce hydrogen.
Applications
- Hydrogen production
- Carbon sequestration - H2 can reduce CO2 to CO, which can be used for feedstock in downstream reactions
- Synthesis of carbon-based chemicals such as:
- syngas
- plastics
- transportation fuel
- Thermal energy storage
Advantages
- Moderate operation temperature:
- thermochemical redox reactions take place at ~ 1200oC or lower
- compatible with existing infrastructure to convert CO and H2 to a variety of chemicals, including liquid fuels
- Scalable - metal oxide materials are in powder form that can be scaled up volumetrically
- Carbon-free pathways:
- thermochemical reaction can be driven by solar power
- unlike SMR, these materials enable H2 production without CO2 waste
- H2 can be used to reduce CO2 to carbon CO for downstream chemical reactions
- High performance material - extremely fast oxygen release kinetics that are comparable to state-of-the art materials such as ceria, even at reduced temperatures
Publications
- Sun, E., Sarkar, A., Gigantino, M., Randall, R., Jaffer, S., Rojas, J., Zhai, S., & Majumdar, A. (2024). Requirements for CO2-free hydrogen production at scale. Joule.
- Rojas, J., Zhai, S., Sun, E., Haribal, V., Marin-Quiros, S., Sarkar, A., Gupta, R., Cargnello, M. , Chueh, W., & Majumdar, A. (2024). Technoeconomics and carbon footprint of hydrogen production. International Journal of Hydrogen Energy, 49, 59-74.
- Zhai, S., Nam, J., Gautam, G. S., Lim, K., Rojas, J., Toney, M. F., Jung, I. H., Chueh, W. C., & Majumdar, A. (2022). Thermodynamic guiding principles of high-capacity phase transformation materials for splitting H 2 O and CO 2 by thermochemical looping. Journal of Materials Chemistry A, 10(7), 3552-3561.
- Ahlborg, N. L., Chueh, W. C., Jin, H., Majumdar, A., Zhai, S., & Herrera, J. A. R. (2021). U.S. Patent No. 10,995,005. Washington, DC: U.S. Patent and Trademark Office.
- Zhai, S., Rojas, J., Ahlborg, N., Lim, K., Cheng, C. H. M., Xie, C., Toney, M. F., Jung, I. H., Chueh, W. C., & Majumdar, A. (2020). High-capacity thermochemical CO 2 dissociation using iron-poor ferrites. Energy & Environmental Science, 13(2), 592-600.
- Zhai, S., Rojas, J., Ahlborg, N., Lim, K., Toney, M. F., Jin, H., Chueh, W.C., & Majumdar, A. (2018). The use of poly-cation oxides to lower the temperature of two-step thermochemical water splitting. Energy & Environmental Science, 11(8), 2172-2178.
Related Links
Patents
- Published Application: 20180118576
- Issued: 10,995,005 (USA)
Similar Technologies
-
Biomimetic Sorbents for CO2 Capture S12-500Biomimetic Sorbents for CO2 Capture
-
Plasmonic gas diffusion reactor for CO2 conversion to high-value chemicals S24-130Plasmonic gas diffusion reactor for CO2 conversion to high-value chemicals
-
High efficiency electrocatalysis with lung-inspired architecture S17-342High efficiency electrocatalysis with lung-inspired architecture