Docket #: S17-207
Stable Lithium Ion Battery Electrodes via Interfacial Layers
Stanford researchers have developed various high ionic conductivity thin films (LiAlO2, LiAlF4) to stabilize lithium ion battery electrodes without sacrificing power density. The atomic layer deposited interfacial layer reduces side reactions between electrolyte and electrode when operated at a wide electrochemical window, maintains power density, and improves energy density – making a safer battery. These thin films are electrochemically inert, chemically stable, lithium ion conductive and can be applied to various battery cathodes.
Stage of Research
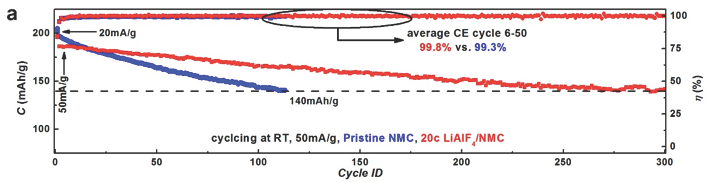
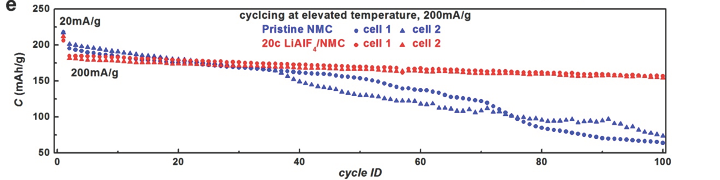
Researchers successfully deposited and tested the interfacial layer on high Ni content, layered lithium transition metal oxides. NMC-811 (LiNi0.8Mn0.1Co0.1O2), a low cost and high capacity electrode, is often limited to narrower electrochemical windows to maintain long-term stability. The LiAlF4 film provided a stable and lithium permeable interfacial layer - stable over 300 cycles with capacity retention higher than 99.9% per cycle at a wide electrochemical window of 2.75-4.50V vs. Li+/Li with a capacity exceeding 140 mAh/g. The LiAlF4 film coated electrode also outperformed uncoated samples at elevated temperatures (50 C) – pristine samples rapidly decayed from 200 mAh/g to less than 100 mAh/g within 100 cycles, while LiAlF4 coated samples maintained capacity exceeding 140 mAh/g within 100 cycles.
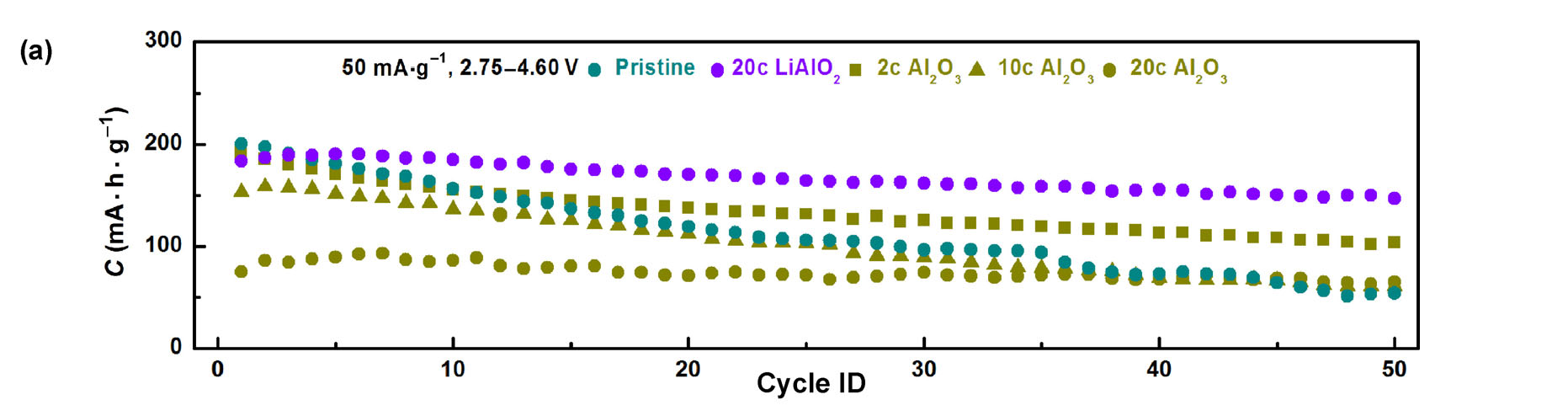
For further demonstration of ALD films, Stanford researchers deposited chemically inert and ionically conductive LiAlO2 on LiCoO2 electrodes by atomic layer deposition. The LiAlO2 coating prevented reactions between the electrode and electrolyte, while allowing lithium ions to freely diffuse into LiCoO2 without sacrificing power density during prolonged high-voltage cycling. The coated electrode's capacity value neared 200 mAh/g for 50 stable cycles with commercial level loading densities (cycled at cut-off potential of 4.6 V vs. Li+/Li) - a 40% capacity gain compared with commercial samples cycled at a cut-off potential of 4.2 V vs. Li+/Li.
Figure 1. (a) Cycle performance of uncoated (pristine) and LiAlF4 coated NMC-811 electrodes at room temperature with an electrochemical window of 2.75-4.50V vs. Li+/Li;
(e) Cycle performance of uncoated (pristine) and LiAlF4 coated NMC-811 electrodes at elevated temperature with an electrochemical window of 2.75-4.50V vs. Li+/Li.
Figure 2. (a) Cycle performance of pristine, 20-cycle-ALD LiAlO2 coated, and 2-, 10-, 20-cycle-ALD Al2O3 coated LiCoO2 electrodes
Applications
- Lithium ion batteries like those used in portable electronics, electrical vehicles, and grid scale power.
Advantages
- Chemically stable, electrochemically inert, lithium ion conductive, and highly uniform cathode.
- Scalable battery production.
Publications
- Cui, Yi and Jin Xie. "Atomic layer deposition of stable lithium ion conductive interfacial layer for stable cathode cycling." WO2018222366 A2, World Intellectual Property Organization, Publication Date 2018-12-06.
- Xie, Jin, Austin D. Sendek, Ekin D. Cubuk, Xiaokun Zhang, Zhiyi Lu, Yongji Gong, Tong Wu et al. "Atomic Layer Deposition of Stable LiAlF4 Lithium Ion Conductive Interfacial Layer for Stable Cathode Cycling." ACS nano 11, no. 7 (2017): 7019-7027.
- Xie, Jin, Jie Zhao, Yayuan Liu, Haotian Wang, Chong Liu, Tong Wu, Po-Chun Hsu, Dingchang Lin, Yang Jin, and Yi Cui. "Engineering the surface of LiCoO2 electrodes using atomic layer deposition for stable high-voltage lithium ion batteries." Nano Research (2017): 1-11.
Related Links
Patents
- Published Application: WO2018222366
- Published Application: 20200152976
- Issued: 11,894,546 (USA)
Similar Technologies
-
Highly Conducting Solid Electrolytes for Batteries S15-203Highly Conducting Solid Electrolytes for Batteries
-
Stabilizing Coating for Lithium Metal Battery Anode S15-283Stabilizing Coating for Lithium Metal Battery Anode
-
Stable Interface for Lithium Batteries via Stitching Two-Dimensional Atomic Crystals by Atomic Layer Deposition S17-198Stable Interface for Lithium Batteries via Stitching Two-Dimensional Atomic Crystals by Atomic Layer Deposition