Docket #: S19-197
Urethral-locating device to assist with female clean intermittent self-catheterization
Stanford researchers have prototyped a medical assistive device which improves efficiency of female self-catheterization by utilizing anatomical landmarks to aid accurate catheter placement in the urethra. Standard of care for patients with neurogenic bladder is clean intermittent self-catheterization where the patient inserts a single-use catheter in the urethra to void their bladder 4-6x a day. By making this process user friendly, this device can mitigate several key UTI risk factors, including bacterial contamination from missing the urethra and inadequate frequency of voiding. Importantly, the device is designed to be easily and reliably used by individuals at home without the need of physician or caregiver assistance.
Please see drawing of prototype below and video demonstrating the mechanism of action in "Related Web Links".
Figure:
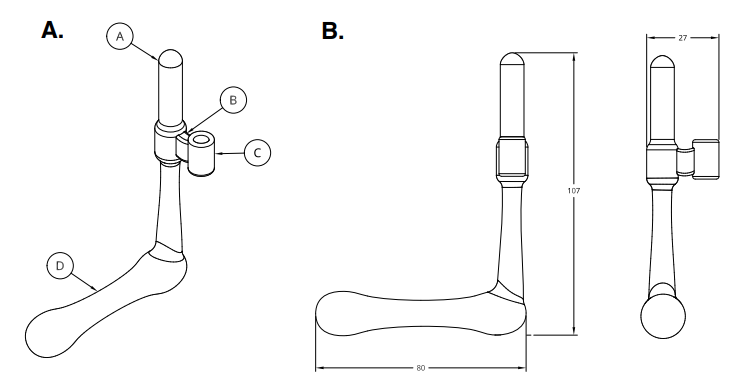
Figure Description: Device components and scale. A. Drawing of current prototype identifying the four components: vaginal insert (A), bridge (B), catheter guide (C), and handle (D). B. Current device prototype dimensions in mm are 80 x 107 x 27.
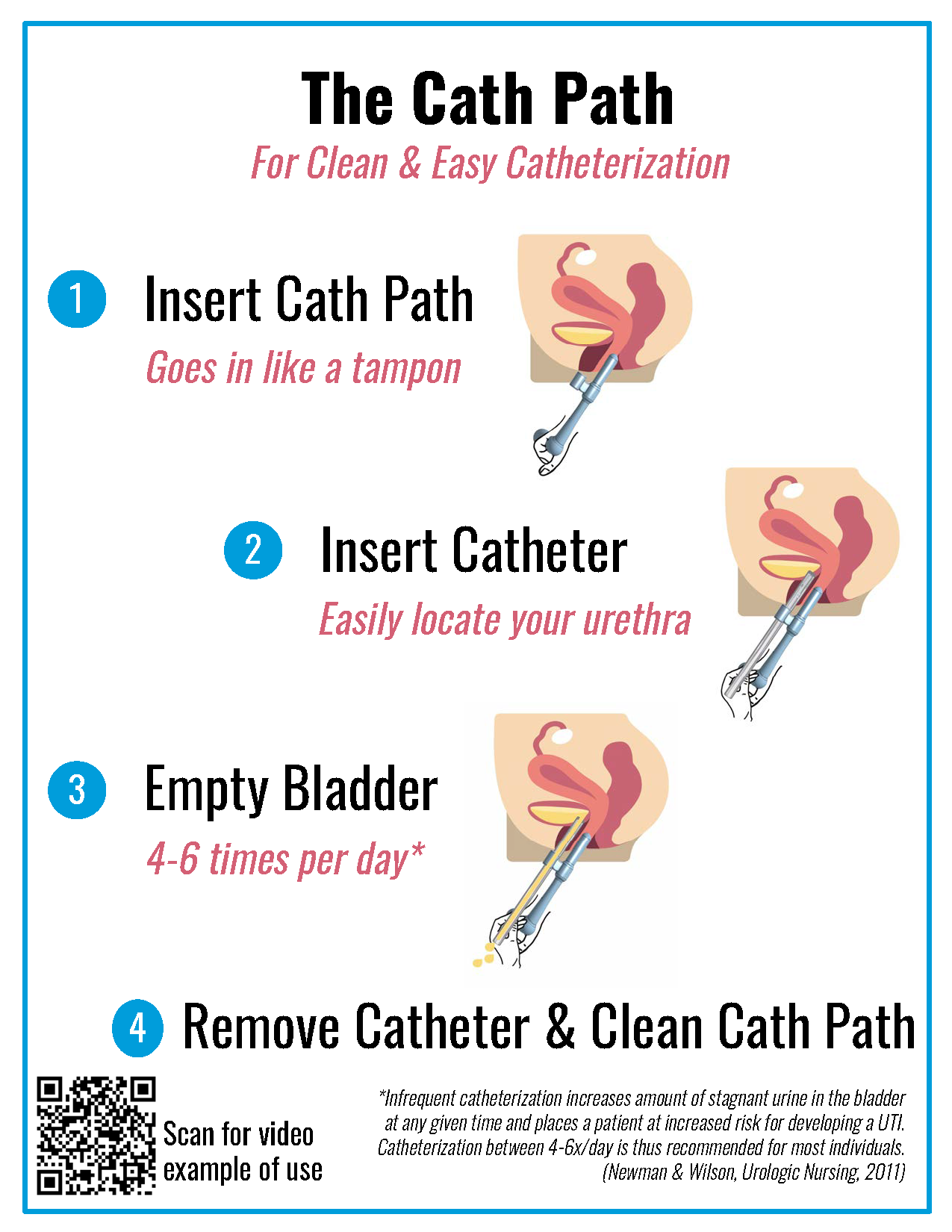
Stage of Development
- Prototypes tested using a novel, non-invasive self-catheterization model.
- IRB protocol has been accepted for review to conduct a 20-patient clinical trial in the near future.
- Device manufacturing for the clinical trial is underway in collaboration with the 3DQ Lab at the Stanford School of Medicine; an autoclavable, biocompatible surgical guide resin was selected for the material.
Applications
- Self-catheterization for females with neurogenic bladder
Advantages
- Improves efficiency of female self-catheterization
- Helps locate and visualize urethra for catheter insertion
- Easier process
- Decreases infection episodes
- More user-friendly than current competitor devices
- Multiple design variations
Related Links
Patents
- Published Application: 20210052852
- Published Application: WO2021041349
- Issued: 11,992,630 (USA)
Similar Technologies
-
Model-less method for controlling continuum and redundant robots S13-079Model-less method for controlling continuum and redundant robots
-
Compact, optical sensor device to measure forces on mitral valve structures prior to surgical repair or replacement S18-298Compact, optical sensor device to measure forces on mitral valve structures prior to surgical repair or replacement
-
A Customizable, Modular Catheter for Hemodialysis Patients of All Ages S20-034A Customizable, Modular Catheter for Hemodialysis Patients of All Ages