Docket #: S11-110
Solution-phase gold films for microarrays and molecular imaging
Researchers in Prof. Hongjie Dai's laboratory have developed a simple process to easily deposit plasmonic nanoscopic gold films on a variety of surfaces. These stable films can enhance the sensitivity and dynamic range of spectroscopic-based biosensing applications by orders of magnitude over unenhanced signals. Because the gold films are a physical enhancement, they do not require additional assay steps. The films can be modified with functional groups or biomolecules and used for a wide variety of research and diagnostic assays. Specific applications of this technology include SERS (surface-enhanced Raman scattering), MEF (metal enhanced fluorescence) in the near-infrared, and NIR-FE (near-infrared fluorescence enhanced) molecular imaging in vitro.
Stage of Research
The inventors have:
- developed the solution-phase, seed-based process and initially demonstrated deposition on a variety of surfaces, including a highly complex surface (protein coated bioassay)
- characterized the nanoscopic structure and localized surface plasmon resonances of such gold films
- used the gold films with SERS to detect a cancer biomarker in serum with a limit of detection ~5fM
- used the gold films with NIR-FE imaging and showed single-molecule imaging and tracking of single-walled nanotubes bound to cellular surface receptors
- used the gold films with MEF protein microarrays, enhancing near-infrared fluorescence ~100-fold, extending the dynamic range of detection by 3 orders of magnitude, to ~ 5fM, and detecting a cancer biomarker in xenograft-bearing mouse serum at ~30fM
- demonstrated proof-of-principle analyte detection in peptide and carbohydrate microarray assays based on MEF of the gold films.
Applications
- Spectroscopic detection in biological assays - for research and diagnostics of diseases such as cancer, viral infection or autoimmunity; assays include:
- SERS - simple process to create gold film for surface-enhanced Raman scattering, for protein, peptide, DNA and carbohydrate sensing and quantification
- MEF - metal enhanced near-infrared fluorophores for protein, peptide, DNA, and carbohydrate microarrays
- NIR-FE - in vitro molecular imaging with near-infrared enhancement using both single-walled carbon nanotube or organic fluorescent labels (particularly for imaging low abundance cell membrane proteins and cell membrane/uptake dynamics)
Advantages
- Simple, facile, solution-phase process - for aqueous phase deposition over a large area on complex surfaces
- Enhanced signal of both fluorescence and Raman scattering in the near infrared, with enhancement factors of 107 observed uniformly and reproducibly (with SERS); 6 - 9x enhancement of molecular imaging (with NIR-FE); 100-fold enhancement of protein detection (with MEF)
- Sensitive - detection limits approximately 1 fM for protein analytes in sandwich assay format
- Broad dynamic range - extended by 3-4 orders of magnitude over unenhanced signal, enabling biological microarrays with ~7 decades of dynamic range
- Simple bioassays - gold film provides physical enhancement and does not require complex assay procedures, specialized equipment, or additional reagents
- Easily modified surface - to present a variety of functional groups or biomolecules
- Variety of surfaces - including glass, quartz, silicon, indium tin oxide, polyvinyl choride, poly dimethylsulfoxide, polystyrene, and amino-modified glass, quartz, or silicon
- Spatial measurements - fluorescence enhancement decays at distances greater than ~50nm, allowing spatial information to be gained based upon degree of fluorescence enhancement in real time without complex optical instrumentation and data processing (with NIR-FE)
Publications
- U.S. Published Patent Application 20130172207, "FLUORESCENCE ENHANCING PLASMONIC NANOSCOPIC GOLD FILMS AND ASSAYS BASED THEREON".
- Guosong Hong, Scott M. Tabakman, Kevin Welsher, Zhuo Chen, Joshua T. Robinson, Hailiang Wang, Bo Zhang and Hongjie Dai, Near-Infrared-Fluorescence-Enhanced Molecular Imaging of Live Cells on Gold Substrates, Angewandte Chemie International Edition, Vol. 50, Issue 20, pp. 4644–4648, May 9, 2011.
- Scott M. Tabakman, Zhuo Chen, Hernan Sanchez Casalongue, Hailiang Wang, Hongjie Dai, A New Approach to Solution-Phase Gold Seeding for SERS Substrates, Small, Vol. 7, Issue 4, pp. 499-505, Feb. 8, 2011 (published online January 3, 2011).
- Scott M. Tabakman, Lana Lau, Joshua T. Robinson, Jordan Price, Sarah P. Sherlock, Hailiang Wang, Bo Zhang, Zhuo Chen, Stephanie Tangsombatvisit, Justin A. Jarrell, Paul J. Utz & Hongjie Dai, Plasmonic substrates for multiplexed protein microarrays with femtomolar sensitivity and broad dynamic range, Nature Communications, published online 13 Sep 2011. DOI: 10.1038/ncomms1477
MEF Microarray Results
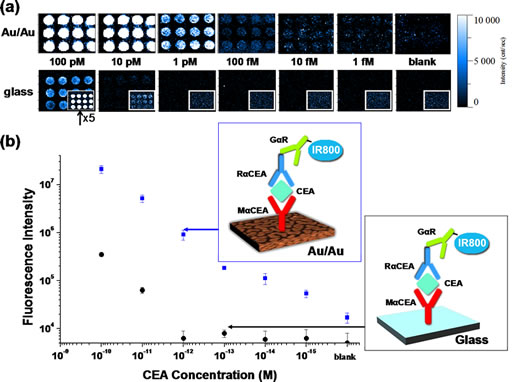
Metal Enhanced Fluorescence-based protein microarrays probed by IR800. (a) gold film (Au/Au) fluorescence maps generated by integration of goat anti-rabbit IgG-IR800 fluorescence emission at 785 nm excitation for different concentrations of the analyte, carcinoembryonic antigen (CEA). Fluorescence maps shown just below are on the same intensity scale for comparison, generated in an identical fashion from a glass substrate (inset shows fluorescence maps with intensity increased 5x). (b) Calibration curves for CEA quantification were generated by averaging the integrated fluorescence intensity of goat anti-rabbit IgG-IR800 emission over the twelve duplicate microarray spots for each CEA concentration on a gold film protein assay as well as a protein microarray on glass as shown in schematics. Error bars represent the standard deviation of the mean over the twelve duplicate assay features.
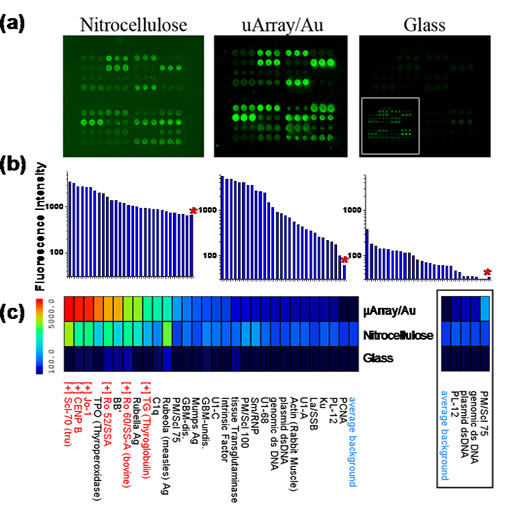
Multiplexed autoantigen array on commercial nitrocellulose, µArray/Au, and glass probed with human serum containing autoantibodies. a) Fluorescence intensity maps generated by scanning IR800-labeled autoantigen/autoantibody arrays with the Licor Odyssey in the 800 nm channel. All images are shown on the same intensity scale, while the scale of the inset in the fluorescence intensity map of glass is increased 10-fold. b) Log scale plot of mean pixel intensity of autoantigen/autoantibody features and average background (denoted by red asterisk) for features printed in triplicate. c) Intensity heatmap comparing the mean feature intensities of the same autoantigens on µArray/Au, nitrocellulose, and glass. “[+]” indicates autoantigens with characterized positive reactivity towards components of the autoimmune serum mixture, shown in red. The simplified heatmap (right, boxed) with identical intensity scale shows only those antigens with low but significant intensity on µArray/Au, yet appear no different than background on nitrocellulose and glass.
Related Links
Patents
- Published Application: 20130172207
- Published Application: 20150226738
Similar Technologies
-
Breakthrough Optical Frequency Processing for Quantum Computing and Beyond S24-365Breakthrough Optical Frequency Processing for Quantum Computing and Beyond
-
Efficient wide-field nanosecond imaging methods using Pockels cells for low-light applications S18-388Efficient wide-field nanosecond imaging methods using Pockels cells for low-light applications
-
Light sheet fluorescence microscopy using high speed structured and pivoting illumination S16-332Light sheet fluorescence microscopy using high speed structured and pivoting illumination